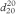Bromine
Br2 = 159.8 (7726-95-6)
Brownish-red fuming liquid, slightly soluble in water, soluble in ethanol (96%).
To prepare 0.05m bromine dissolve 3 g of potassium bromate and 5 g of potassium bromide in sufficient water to produce 1000 mL. Weaker solutions should be prepared using proportionately lesser amounts of reagents or by appropriate dilution.