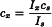Appendix II E. Fluorescence Spectrophotometry [Fluorimetry]
Fluorimetry is a procedure which uses the measurement of the intensity of the fluorescent light emitted by the substance to be examined in relation to that emitted by a given standard.
Method Dissolve the substance to be examined in the solvent or mixture of solvents prescribed in the monograph, transfer the solution to the cell or the tube of the fluorimeter and illuminate it with an excitant light beam of the wavelength prescribed in the monograph and as near as possible monochromatic.
Measure the intensity of the emitted light at an angle of 90° to the excitant beam, after passing it through a filter which transmits predominantly light of the wavelength of the fluorescence. Other types of apparatus may be used provided that the results obtained are identical.
For quantitative determinations, first introduce into the apparatus the solvent or mixture of solvents used to dissolve the substance to be examined and set the instrument to zero. Introduce the standard solution and adjust the sensitivity of the instrument so that the reading is greater than 50. If the second adjustment is made by altering the width of the slits, a new zero setting must be made and the intensity of the standard must be measured again. Finally introduce the solution of unknown concentration and read the result on the instrument. Calculate the concentration cx of the substance in the solution to be examined, using the formula:
| cx | = | concentration of the solution to be examined, |
| cs | = | concentration of the standard solution, |
| Ix | = | intensity of the light emitted by the solution to be examined, |
| Is | = | intensity of the light emitted by the standard solution. |
If the intensity of the fluorescence is not strictly proportional to the concentration, the measurement may be effected using a calibration curve.
In some cases, measurement can be made with reference to a fixed standard (for example a fluorescent glass or a solution of another fluorescent substance). In such cases, the concentration of the substance to be examined must be determined using a previously drawn calibration curve under the same conditions.