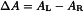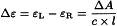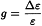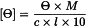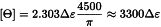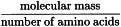Appendix V J. Circular Dichroism
The difference in absorbance of optically active substances within an absorption band for left and right circularly polarised light is referred to as circular dichroism.
Direct measurement gives a mean algebraic value:
ΔA | = | circular dichroic absorbance, |
AL | = | absorbance of left circularly polarised light, |
AR | = | absorbance of right circularly polarised light. |
Circular dichroism is calculated using the equation:
Δε | = | molar circular dichroism or molar differential dichroic absorptivity expressed in litre⋅mole-1⋅cm-1, |
εL | = | molar absorptivity (2.2.25) of left circularly polarised light, |
εR | = | molar absorptivity of right circularly polarised light, |
c | = | concentration of the test solution in mole⋅litre-1, |
l | = | optical path of the cell in centimetres. |
The following units may also be used to characterise circular dichroism:
ε | = | molar absorptivity (2.2.25). |
Certain types of instruments display directly the value of ellipticity Θ, expressed in degrees. When such instruments are used, the molar ellipticity [Θ] may be calculated using the following equation:
[Ө] | = | molar ellipticity, expressed in degrees⋅cm2⋅decimole-1, |
Ө | = | value of ellipticity given by the instrument, |
M | = | relative molecular mass of the substance to be examined, |
c | = | concentration of the solution to be examined in g/mL, |
l | = | optical path of the cell in centimetres. |
Molar ellipticity is also related to molar circular dichroism by the following equation:
Molar ellipticity is often used in the analysis of proteins and nucleic acids. In this case, molar concentration is expressed in terms of monomeric residue, calculated using the expression:
The mean relative molecular mass of the monomeric residue is 100 to 120 (generally 115) for proteins and about 330 for nucleic acids (as the sodium salt).
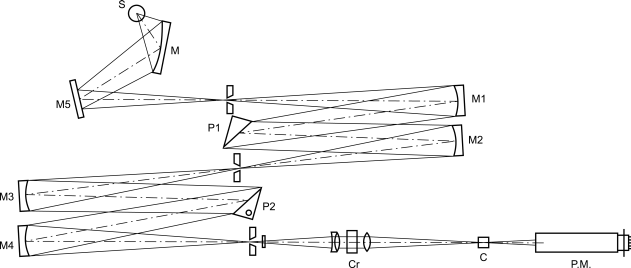
Apparatus
The light source (S) is a xenon lamp (Figure 2.2.41.-1); the light passes through a double monochromator (M) equipped with quartz prisms (P1, P2).
The linear beam from the first monochromator is split into 2 components polarised at right angles in the second monochromator. The exit slit of the monochromator eliminates the extraordinary beam.
The polarised and monochromatic light passes through a birefringent modulator (Cr): the result is alternating circularly polarised light.
The beam then passes through the sample to be examined (C) and reaches a photomultiplier (PM) followed by an amplifier circuit which produces 2 electrical signals: one is a direct current Vc and the other is an alternating current at the modulation frequency Vac characteristic of the sample to be examined. The phase gives the sign of the circular dichroism. The ratio Vac/Vc is proportional to the differential absorption ΔA which created the signal. The region of wavelengths normally covered by a dichrograph is 170 nm to 800 nm.
Calibration of the apparatus
Accuracy of absorbance scale Dissolve 10.0 mg of isoandrosterone R in dioxan R and dilute to 10.0 mL with the same solvent. Record the circular dichroism spectrum of the solution between 280 nm and 360 nm. Measured at the maximum at 304 nm, Δε is + 3.3.
The solution of (1S)-(+)-10-camphorsulfonic acid R may also be used.
Linearity of modulation Dissolve 10.0 mg of (1S)-(+)-10-camphorsulfonic acid R in water R and dilute to 10.0 mL with the same solvent. Determine the exact concentration of camphorsulfonic acid in the solution by ultraviolet spectrophotometry (2.2.25), taking the specific absorbance to be 1.49 at 285 nm.
Record the circular dichroism spectrum between 185 nm and 340 nm. Measured at the maximum at 290.5 nm, Δε is + 2.2 to + 2.5. Measured at the maximum at 192.5 nm, Δε is -4.3 to -5.
(1)-(+)-S Or antipodal (1R)-(-)-ammonium 10-camphorsulfonate R can also be used.