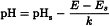Appendix V L. Determination of pH Values
The pH of an aqueous solution is defined as the negative logarithm of the activity of its hydrogen ions, expressed conventionally as the hydrogen ion concentration of the solution. For practical purposes, its definition is an experimental one. The pH of a solution to be examined is related to that of a reference solution (pHs) by the following equation:
in which E is the potential, expressed in volts, of the cell containing the solution to be examined and Es is the potential, expressed in volts, of the cell containing the solution of known pH (pHs), k is the change in potential per unit change in pH, expressed in volts and calculated from the Nernst equation.
The potentiometric determination of pH is made by measuring the potential difference between 2 appropriate electrodes immersed in the solution to be examined; 1 of these electrodes is sensitive to hydrogen ions (usually a glass electrode) and the other is the reference electrode (e.g. a silver-silver chloride electrode). They are often combined as 1 compact electrode, together with a temperature probe.
Apparatus The measuring apparatus is usually a voltmeter with an input resistance at least 100 times that of the electrodes used. It is normally graduated in pH units and has a sensitivity such that discrimination of at least 0.05 pH unit or at least 0.003 V may be achieved.
Recent pH meters are microprocessor-controlled and are operated using the manufacturer′s firmware or software, according to given instructions.
Management of electrodes The electrodes are stored appropriately and according to the manufacturer′s recommendations (e.g. in an electrolyte solution or a suitable storage solution). Before measurement, the electrodes are visually checked. Refillable electrodes are checked for the absence of air bubbles in the glass bulb and to ensure that the inner electrolyte solution level is satisfactory. The filling orifice has to remain open during the measurement. It is also recommended that the diaphragm of the reference electrode is checked. Before first use, or if the electrode has been stored out of electrolyte solution, it is usually necessary to condition it, according to the recommendations of the manufacturer. If pH stabilisation is too slow (i.e. a long response time), or a zero point shift, reduced slope or any other difficulties in calibration are observed, the electrode will probably need to be cleaned or replaced. The cleaning is performed depending on the type of sample and as prescribed in the manufacturer′s manual. Regular cleaning is recommended.
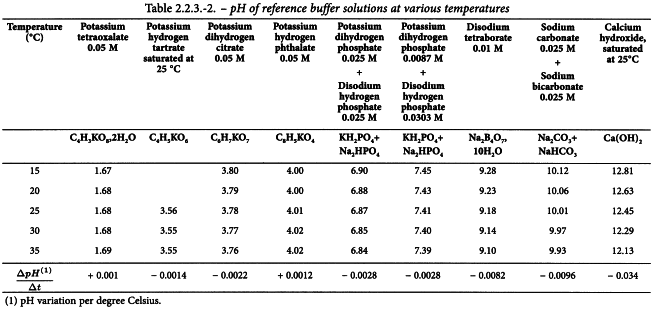
Calibration and measurement conditions Unless otherwise prescribed in the monograph, all measurements are carried out at the same temperature as that used for calibration (± 2.5 °C), usually between 20 °C and 25 °C. Table 2.2.3.-2 shows the variation of pH with respect to temperature of a number of reference buffer solutions used for calibration. Follow the manufacturer′s instructions for temperature correction.
The calibration consists of the determination of the slope (e.g. 95-105 per cent) and the offset of the measuring system. Most commercial pH meters offer a “self test” or “start-up test” where, for example, the slope and asymmetry potential are tested and compared to the manufacturer‘s specifications. The apparatus is calibrated using at least 2 buffer solutions chosen so that the expected pH value of the solution to be examined lies between the pH values of the buffer solutions. The range must be at least 2 pH units. The pH of another buffer solution of intermediate pH, read from the scale, must not differ by more than 0.05 pH units from the value corresponding to that solution.
Reference buffer solutions are preferably commercially available certified reference materials. Alternatively, buffer solutions can be prepared in-house according to Table 2.2.3.-2. These solutions must be traceable to primary standards. Calibration has to be performed on a regular basis, preferably each day of use or before each series of measurements.
Immerse the electrodes in the solution to be examined and take the reading in the same conditions as those applied for the reference buffer solutions.
If suspensions, emulsions or solutions of non-aqueous or partially non-aqueous character are measured on a system calibrated as described above, the pH reading can only be considered to be an approximation of the true value. Suitable electrodes have to be used for pH measurements of such mixtures.
PREPARATION OF REFERENCE BUFFER SOLUTIONS
Potassium tetraoxalate 0.05 M
Dissolve 12.61 g of C4H3KO8,2H2O in carbon dioxide-free water R and dilute to 1000.0 mL with the same solvent.
Potassium hydrogen tartrate, saturated at 25 °C
Shake an excess of C4H5KO6 vigorously with carbon dioxide-free water R at 25 °C. Filter or decant. Prepare immediately before use.
Potassium dihydrogen citrate 0.05 M
Dissolve 11.41 g of C6H7KO7 in carbon dioxide-free water R and dilute to 1000.0 mL with the same solvent. Prepare immediately before use.
Potassium hydrogen phthalate 0.05 M
Dissolve 10.13 g of C8H5KO4, previously dried for 1 h at 110 ± 2 °C, in carbon dioxide-free water R and dilute to 1000.0 mL with the same solvent.
Potassium dihydrogen phosphate 0.025 M + Disodium hydrogen phosphate 0.025 M
Dissolve 3.39 g of KH2PO4 and 3.53 g of Na2HPO4, both previously dried for 2 h at 120 ± 2 °C, in carbon dioxide-free water R and dilute to 1000.0 mL with the same solvent.
Potassium dihydrogen phosphate 0.0087 M + Disodium hydrogen phosphate 0.0303 M
Dissolve 1.18 g of KH2PO4 and 4.30 g of Na2HPO4, both previously dried for 2 h at 120 ± 2 °C, in carbon dioxide-free water R and dilute to 1000.0 mL with the same solvent.
Disodium tetraborate 0.01 M
Dissolve 3.80 g of Na2B4O7,10H2O in carbon dioxide-free water R and dilute to 1000.0 mL with the same solvent. Store protected from atmospheric carbon dioxide.
Sodium carbonate 0.025 M + Sodium hydrogen carbonate 0.025 M
Dissolve 2.64 g of Na2CO3 and 2.09 g of NaHCO3 in carbon dioxide-free water R and dilute to 1000.0 mL with the same solvent. Store protected from atmospheric carbon dioxide.
Calcium hydroxide, saturated at 25 °C
Shake an excess of calcium hydroxide R with carbon dioxide-free water R and decant at 25 °C. Store protected from atmospheric carbon dioxide.
STORAGE of buffer solutions
Store buffer solutions in suitable chemically-resistant, airtight containers, such as type I glass bottles or plastic containers suitable for aqueous solutions.
Additional points for monographs of the British Pharmacopoeia
[Suitable glass electrodes and pH meters of both the analogue and digital type are described in British Standards 2586:1979 and 3145:1978.]