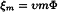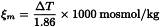Appendix V N. Osmolality
Osmolality is a practical means of giving an overall measure of the contribution of the various solutes present in a solution to the osmotic pressure of the solution.
An acceptable approximation for the osmolality ξm of a given aqueous solution is given by:
If the solute is not ionised, υ = 1; otherwise υ is the total number of ions already present or formed by solvolysis from 1 molecule of solute.
| m | = | molality of the solution, that is the number of moles of solute per kilogram of solvent; |
| Φ | = | molal osmotic coefficient which takes account of the interactions between ions of opposite charge in the solution. It is dependent on the value of m. As the complexity of solutions increases, Φ becomes difficult to measure. |
The unit of osmolality is osmole per kilogram (osmol/kg), but the submultiple milliosmole per kilogram (mosmol/kg) is usually used.
Unless otherwise prescribed, osmolality is determined by measurement of the depression of freezing point. The following relationship exists between the osmolality and the depression of freezing point ΔT:
Apparatus The apparatus (osmometer) consists of:
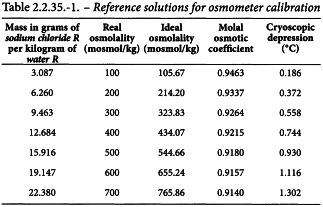
Method Prepare reference solutions as described in Table 2.2.35.-1, as required. Determine the zero of the apparatus using water R. Calibrate the apparatus using the reference solutions: introduce a suitable volume of sample into the measurement cell, as indicated by the equipment supplier, and start the cooling system. Usually, the mixing device is programmed to operate at a temperature below that expected through cryoscopic depression to prevent supercooling. A suitable device indicates attainment of equilibrium. Before each measurement, rinse the measurement cell with the solution to be examined.
Carry out the same operations with the test sample. Read directly the osmolality or calculate it from the measured depression of freezing point. The test is not valid unless the value found is within 2 values of the calibration scale.