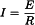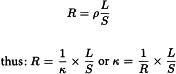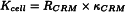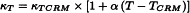Appendix V O. Conductivity
The current I (in amperes) flowing in a conductor is directly proportional to the applied electromotive force E (in volts) and inversely proportional to the resistance R (in ohms) of the conductor:
The conductivity (formerly called specific conductance) of a solution (κ) is, by definition, the reciprocal of resistivity (ρ). Resistivity is defined as the quotient of the electric field and the density of the current. The resistance R (in Ω) of a conductor of cross-section S (in cm2) and length L (in cm) is given by the expression:
L/S Corresponds to the ideal cell constant.
The unit of conductivity in the International System is the siemens per metre (S⋅m-1). In practice, the electrical conductivity of a solution is expressed in siemens per centimetre (S⋅cm-1) or in microsiemens per centimetre (µS⋅cm-1). The unit of resistivity in the International System is the ohm-metre (Ω⋅m). The resistivity of a solution is generally expressed in ohm-centimetres (Ω⋅cm). Unless otherwise prescribed, the reference temperature for the expression of conductivity or resistivity is 25 °C.
The apparatus and operating procedure described below are applicable to laboratory measurement of conductivity greater than 10 µS⋅cm-1. The measurement of conductivity of water is dealt with in the relevant monographs.
APPARATUS
The apparatus used (conductivity meter or resistivity meter) measures the resistance of the column of liquid between the electrodes of the immersed measuring device (conductivity cell). The apparatus is supplied with alternating current to avoid the effects of electrode polarisation. It is equipped with a temperature probe and a temperature compensation device.
The conductivity cell contains 2 parallel platinum electrodes coated with platinum black, each with a surface area S, and separated from the other by a distance L. Both are generally protected by a glass tube. Other types of cells may also be used.
OPERATING PROCEDURE
Determination of the cell constant
Choose a conductivity cell that is appropriate for the properties and conductivity of the solution to be examined. The higher the expected conductivity, the higher the cell constant that must be chosen (low ρ). Commonly used conductivity cells have cell constants of the order of 0.1 cm-1, 1 cm-1 and 10 cm-1. Use a certified reference material, for example a solution of potassium chloride, that is appropriate for the measurement. The conductivity value of the certified reference material, should be near the expected conductivity value of the solution to be examined. Other certified reference materials may be used especially for cells having a constant of 0.1 cm-1. Rinse the cell several times with distilled water R and at least twice with the certified reference material used for the determination of the cell constant of the conductivity cell. Measure the resistance of the conductivity cell using the certified reference material at 25 ± 1 °C. The cell constant Kcell (in cm-1) depends on the geometry of the conductivity cell and is given by the expression:
| RCRM | = | measured resistance, expressed in mega-ohms, |
| κCRM | = | conductivity of the certified reference material solution used, expressed in microsiemens per centimetre. |
The measured constant Kcell of the conductivity cell must be within 5 per cent of the value indicated.
If the determination of the cell constant is carried out at a different temperature than that indicated for the certified reference material, the conductivity value may be calculated from the following expression:
| κT | = | value of conductivity at the different temperature, |
| κTCRM | = | value of conductivity of the certified reference material, |
| T | = | temperature set for calibration, |
| TCRM | = | temperature indicated for the certified reference material, |
| α | = | temperature coefficient for the conductivity value of the certified reference material; for potassium chloride α = 0.021. |
Determination of the conductivity of the solution to be examined
After calibrating the apparatus with a certified reference material solution, rinse the conductivity cell several times with distilled water R and at least twice with the aqueous solution to be examined. Carry out successive measurements as described in the monograph.