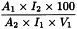Appendix VIII F. Determination of Ethanol
Use Method I or, where appropriate, Method II unless otherwise prescribed in the monograph.
Method I
Carry out the method for gas chromatography, Appendix III B, using the following solutions. Solution (1) contains 5.0% v/v of absolute ethanol and 5.0% v/v of propan-1-ol (internal standard). For solution (2) dilute a volume of the preparation being examined with water to contain between 4.0 and 6.0% v/v of ethanol. Prepare solution (3) in the same manner as solution (2) but adding sufficient of the internal standard to produce a final concentration of 5.0% v/v.
The chromatographic procedure may be carried out using a column (1.5 m × 4 mm) packed with porous polymer beads (100 to 120 mesh) (Porapak Q and Chromosorb 101 are suitable) and maintained at 150° with both the inlet port and the detector at 170°.
Calculate the percentage content of ethanol from the areas of the peaks due to ethanol in the chromatograms obtained with solutions (1) and (3).
Method II
For preparations in which, in accordance with the authority given in the monographs, Industrial Methylated Spirit has been used, determine the content of ethanol as described in Method I but using as solution (2) a volume of the preparation being examined diluted with water to contain between 4.0 and 6.0% v/v of total ethanol and methanol.
Determine the concentration of methanol in the following manner. Carry out the chromatographic procedure described under Method I but using the following solutions. Solution (1) contains 0.25% v/v of methanol and 0.25% v/v of propan-1-ol (internal standard). For solution (2) dilute a volume of the preparation being examined with water to contain between 0.2% and 0.3% v/v of methanol. Prepare solution (3) in the same manner as solution (2) but adding sufficient of the internal standard to produce a final concentration of 0.25% v/v.
The sum of the contents of ethanol and methanol is within the range specified in the monograph and the ratio of the content of methanol to that of ethanol is commensurate with Industrial Methylated Spirit having been used.
Method III
Method A
Where preparations contain dissolved substances, the dissolved substances must be separated from the ethanol that is to be determined by distillation. Where distillation would distil volatile substances other than ethanol and water, the appropriate precautions are stated in the monograph.
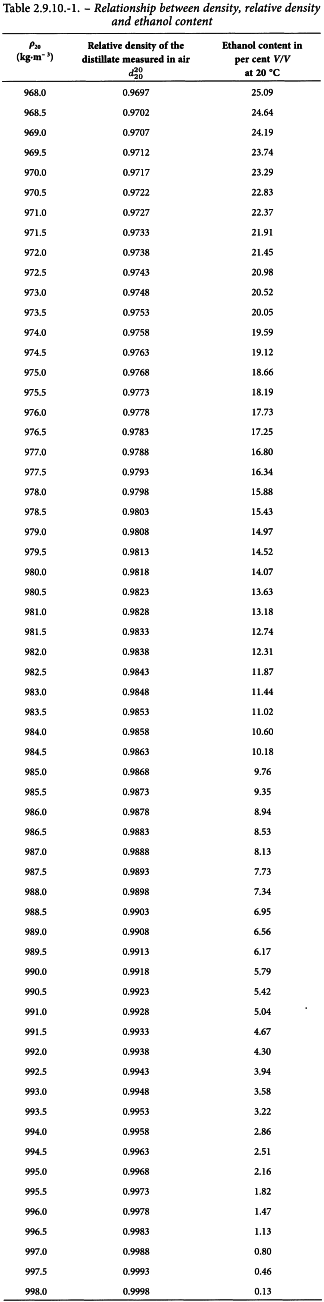
The relation between the density at 20 ± 0.1 °C, the relative density (corrected to vacuum) and the ethanol content of a mixture of water and ethanol is given in the tables of the International Organisation for Legal Metrology (1972), International Recommendation No. 22.
Apparatus
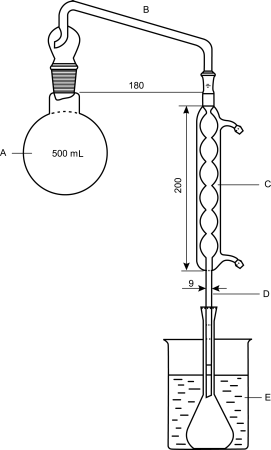
The apparatus (see Figure 2.9.10.-1) consists of a round-bottomed flask (A) fitted with a distillation head (B) with a steam trap and attached to a vertical condenser (C). The latter is fitted at its lower part with a tube (D), which carries the distillate into the lower part of a 100 mL or 250 mL volumetric flask. The volumetric flask is immersed in a mixture of ice and water (E) during the distillation. A disc having a circular aperture 6 cm in diameter is placed under the flask (A) to reduce the risk of charring any dissolved substances.
Method
Pycnometer method/oscillating transducer density meter method Transfer 25.0 mL of the preparation to be examined, measured at 20 ± 0.1 °C, to the distillation flask. Dilute with 100-150 mL of distilled water R and add a few pieces of pumice. Attach the distillation head and condenser. Distil and collect not less than 90 mL of distillate in a 100 mL volumetric flask. Adjust the temperature to 20 ± 0.1 °C and dilute to 100.0 mL with distilled water R at 20 ± 0.1 °C. Determine the relative density at 20 ± 0.1 °C using a pycnometer or an oscillating transducer density meter.
The values indicated in Table 2.9.10.-1, column 3, are multiplied by 4 to obtain the percentage of ethanol by volume (V/V) contained in the preparation. After calculation of the ethanol content using the table, round off the result to 1 decimal place.
Hydrometer method Transfer 50.0 mL of the preparation to be examined, measured at 20 ± 0.1 °C, to the distillation flask, add 200-300 mL of distilled water R and distil, as described above, into a volumetric flask until at least 180 mL has been collected. Adjust the temperature to 20 ± 0.1 °C and dilute to 250.0 mL with distilled water R at 20 ± 0.1 °C.
Transfer the distillate to a cylinder whose diameter is at least 6 mm wider than the bulb of the hydrometer. If the volume is insufficient, double the quantity of the sample and dilute the distillate to 500.0 mL with distilled water R at 20 ± 0.1 °C.
Multiply the strength by 5 to allow for the dilution during the determination. After calculation of the ethanol content using Table 2.9.10.-1, round off the result to 1 decimal place.
Method B
Head-space gas chromatography (2.2.28).
Internal standard solution Dilute 1.0 mL of propanol R1 to 100.0 mL with water R. Dilute 1.0 mL of the solution to 20.0 mL with water R.
Test solution Dilute a volume of the preparation to be examined corresponding to 0.4 g of ethanol to 50.0 mL with water R. Dilute 1.0 mL of the solution to 20.0 mL with water R. To 2.0 mL of this solution add 1.0 mL of the internal standard solution and dilute to 20.0 mL with water R.
Reference solution (a) Dilute 5.0 mL of anhydrous ethanol R to 100.0 mL with water R. Dilute 25.0 mL of the solution to 100.0 mL with water R. Dilute 1.0 mL of this solution to 20.0 mL with water R.
Reference solution (b) Mix 0.5 mL of reference solution (a) and 1.0 mL of the internal standard solution and dilute to 20.0 mL with water R.
Reference solution (c) Mix 1.0 mL of reference solution (a) and 1.0 mL of the internal standard solution and dilute to 20.0 mL with water R.
Reference solution (d) Mix 1.5 mL of reference solution (a) and 1.0 mL of the internal standard solution and dilute to 20.0 mL with water R.
Reference solution (e) Dilute 1.0 mL of methanol R2 to 100.0 mL with water R. Dilute 1.0 mL of the solution to 20.0 mL with water R.
Reference solution (f) Mix 1.0 mL of the internal standard solution, 2.0 mL of reference solution (a) and 2.0 mL of reference solution (e) and dilute to 20.0 mL with water R.
Carrier gas helium for chromatography R.
Flow rate 3 mL/min.
Split ratio 1:50.
Detection Flame ionisation.
Injection 1.0 mL of the gaseous phase of the test solution and reference solutions (b), (c), (d) and (f), at least 3 times.
Elution order Methanol, ethanol, propanol.
Relative retention With reference to ethanol (retention time = about 5.3 min): methanol = about 0.8; propanol = about 1.6.
Establish a calibration curve with the concentration of ethanol in reference solutions (b), (c), (d) and (f) as the abscissa and the mean ratio of the peak area of ethanol to the peak area of the internal standard in the corresponding chromatograms as the ordinate.
Calculate the percentage content of ethanol in the preparation to be examined.
Method C
Internal standard solution Dilute 1.0 mL of propanol R1 to 100.0 mL with water R.
Test solution Dilute a volume of the preparation to be examined corresponding to 1 g of ethanol to 50.0 mL with water R. To 1.0 mL of this solution add 1.0 mL of the internal standard solution and dilute to 20.0 mL with water R.
Reference solution (a) Dilute 1.0 mL of anhydrous ethanol R to 50.0 mL with water R.
Reference solution (b) Dilute 1.0 mL of methanol R2 to 100.0 mL with water R. Dilute 1.0 mL of the solution to 20.0 mL with water R.
Reference solution (c) Mix 1.0 mL of the internal standard solution, 1.0 mL of reference solution (a) and 2.0 mL of reference solution (b) and dilute to 20.0 mL with water R.
Carrier gas helium for chromatography R.
Flow rate 3 mL/min.
Split ratio 1:50.
Detection Flame ionisation.
Injection 1.0 µL of the test solution and reference solution (c), at least 3 times.
Elution order Methanol, ethanol, propanol.
Relative retention With reference to ethanol (retention time = about 5.3 min): methanol = about 0.8; propanol = about 1.6.
Calculate the ethanol content in per cent V/V using the following expression:
A1 | = | area of the peak due to ethanol in the chromatogram obtained with the test solution; |
A2 | = | area of the peak due to ethanol in the chromatogram obtained with reference solution (c); |
I1 | = | area of the peak due to the internal standard in the chromatogram obtained with the test solution; |
I2 | = | area of the peak due to the internal standard in the chromatogram obtained with reference solution (c); |
V1 | = | volume of the preparation to be examined in the test solution, in millilitres. |