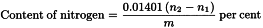Appendix VIII H. Determination of Nitrogen
Method I
SEMI-MICRO METHOD
Place a quantity of the substance to be examined (m g) containing about 2 mg of nitrogen in a combustion flask, add 4 g of a powdered mixture of 100 g of dipotassium sulfate R, 5 g of copper sulfate pentahydrate R and 2.5 g of selenium R, and three glass beads. Wash any adhering particles from the neck into the flask with 5 mL of sulfuric acid R, allowing it to run down the sides of the flask, and mix the contents by rotation. Close the mouth of the flask loosely, for example by means of a glass bulb with a short stem, to avoid excessive loss of sulfuric acid. Heat gradually at first, then increase the temperature until there is vigorous boiling with condensation of sulfuric acid in the neck of the flask; precautions should be taken to prevent the upper part of the flask from becoming overheated. Continue the heating for 30 min, unless otherwise prescribed. Cool, dissolve the solid material by cautiously adding to the mixture 25 mL of water R, cool again and place in a steam-distillation apparatus. Add 30 mL of strong sodium hydroxide solution R and distil immediately by passing steam through the mixture. Collect about 40 mL of distillate in 20.0 mL of 0.01 M hydrochloric acid and enough water R to cover the tip of the condenser. Towards the end of the distillation, lower the receiver so that the tip of the condenser is above the surface of the acid. Take precautions to prevent any water on the outer surface of the condenser from reaching the contents of the receiver. Titrate the distillate with 0.01 M sodium hydroxide, using methyl red mixed solution R as indicator (n1 mL of 0.01 M sodium hydroxide).
Repeat the test using about 50 mg of glucose R in place of the substance to be examined (n2 mL of 0.01 M sodium hydroxide).
Method II (Determination of Protein in Blood Products)
For dried blood products prepare a solution of the preparation as directed in the monograph.
To a volume expected to contain about 0.1 g of protein add sufficient saline solution to produce 20 mL. To 2 mL of the resulting solution, in a 75-mL boiling tube, add 2 mL of a solution containing 75% v/v of nitrogen-free sulfuric acid, 4.5% w/v of potassium sulfate and 0.5% w/v of copper(ii) sulfate, mix and loosely stopper the tube. Heat gradually to boiling, boil vigorously for 1.5 hours and cool. If the solution is not clear add 0.25 mL of hydrogen peroxide solution (20 vol), continue heating until a clear solution is produced and cool. During heating, take precautions to ensure that the upper part of the tube is not overheated.
Transfer the solution to a distillation apparatus using three 3-mL quantities of water, add 10 mL of 10m sodium hydroxide and distil rapidly for 4 minutes, collecting the distillate in a mixture of 5 mL of a saturated solution of boric acid and 5 mL of water and keeping the tip of the condenser below the level of the acid. Lower the collection flask so that the condenser can drain freely and continue the distillation for a further 1 minute. Titrate with 0.02m hydrochloric acid VS using methyl red mixed solution as indicator (V1 mL).
To a further volume of the preparation being examined, or of the solution prepared from it, expected to contain about 0.1 g of protein, add 12 mL of saline solution, 2 mL of a 7.5% w/v solution of sodium molybdate and 2 mL of a mixture of 1 volume of nitrogen-free sulfuric acid and 30 volumes of water. Shake, allow to stand for 15 minutes, add sufficient water to produce 20 mL, shake again and centrifuge. Using 2 mL of the resulting clear supernatant liquid repeat the procedure described above beginning at the words ‘in a 75-mL boiling tube…’ (V2 mL). Calculate the protein content in mg per mL of the preparation being examined, using the expression 6.25 × 0.280(V1–V2) and taking into account the initial dilution.