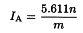Appendix X B. Acid Value
The acid value IA is the number that expresses, in milligrams the quantity of potassium hydroxide required to neutralise the free acids present in 1 g of the substance.
Dissolve 10.00 g of the substance to be examined, or the quantity prescribed, (m g), in 50 mL of a mixture of equal volumes of ethanol (96 per cent) R and light petroleum R3, previously neutralised with 0.1 M potassium hydroxide or 0.1 M sodium hydroxide, unless otherwise specified, using 0.5 mL of phenolphthalein solution R1 as indicator. If necessary, heat to about 90 °C to dissolve the substance to be examined. When the substance to be examined has dissolved, titrate with 0.1 M potassium hydroxide or 0.1 M sodium hydroxide until the pink colour persists for at least 15 s (n mL of titrant). When heating has been applied to aid dissolution, maintain the temperature at about 90 °C during the titration.