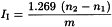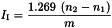Appendix X E. Iodine Value
The iodine value II is the number that expresses in grams the quantity of halogen, calculated as iodine, that can be fixed in the prescribed conditions by 100 g of the substance.
When the monograph does not specify the method to be used, method A is applied. Any change from method A to method B is validated.
METHOD A
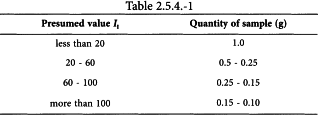
Unless otherwise prescribed, use the following quantities (Table 2.5.4.-1) for the determination.
Introduce the prescribed quantity of the substance to be examined (m g) into a 250 mL flask fitted with a ground-glass stopper and previously dried or rinsed with glacial acetic acid R, and dissolve it in 15 mL of chloroform R unless otherwise prescribed. Add very slowly 25.0 mL of iodine bromide solution R. Close the flask and keep it in the dark for 30 min unless otherwise prescribed, shaking frequently. Add 10 mL of a 100 g/L solution of potassium iodide R and 100 mL of water R. Titrate with 0.1 M sodium thiosulfate, shaking vigorously until the yellow colour is almost discharged. Add 5 mL of starch solution R and continue the titration adding the 0.1 M sodium thiosulfate dropwise until the colour is discharged (n1 mL of 0.1 M sodium thiosulfate). Carry out a blank test under the same conditions (n2 mL of 0.1 M sodium thiosulfate).
METHOD B
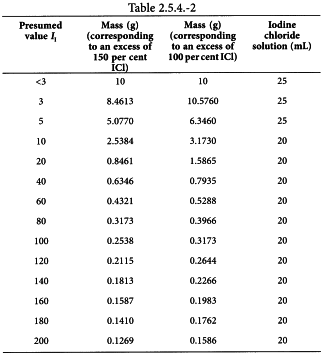
Unless otherwise prescribed, use the following quantities (Table 2.5.4.-2) for the determination.
The mass of the sample is such that there will be an excess of iodine chloride solution R of 50 per cent to 60 per cent of the amount added, i.e. 100 per cent to 150 per cent of the amount absorbed.
Introduce the prescribed quantity of the substance to be examined (m g) into a 250 mL flask fitted with a ground-glass stopper and previously rinsed with glacial acetic acid R or dried, and dissolve it in 15 mL of a mixture of equal volumes of cyclohexane R and glacial acetic acid R, unless otherwise prescribed. If necessary, melt the substance before dissolution (melting point greater than 50 °C). Add very slowly the volume of iodine chloride solution R stated in Table 2.5.4.-2. Close the flask and keep it in the dark for 30 min, unless otherwise prescribed, shaking frequently. Add 10 mL of a 100 g/L solution of potassium iodide R and 100 mL of water R. Titrate with 0.1 M sodium thiosulfate, shaking vigorously until the yellow colour is almost discharged. Add 5 mL of starch solution R and continue the titration adding the 0.1 M sodium thiosulfate dropwise until the colour is discharged (n1 mL of 0.1 M sodium thiosulfate). Carry out a blank test under the same conditions (n2 mL of 0.1 M sodium thiosulfate).
Iodine Monochloride Method
When the use of iodine flasks is prescribed, use flasks with a nominal capacity of 250 mL and complying with British Standard 2735:1956 (Specification for iodine flasks), unless otherwise specified.
Dissolve the specified quantity of the substance being examined in 10 mL of dichloromethane in a dry iodine flask. Add 20 mL of iodine monochloride solution, insert the stopper, previously moistened with dilute potassium iodide solution, and allow to stand in the dark at 15° to 25° for 30 minutes. Place 15 mL of dilute potassium iodide solution in the top cup, carefully remove the stopper, rinse the stopper and the sides of the flask with 100 mL of water, shake and titrate with 0.1m sodium thiosulfate VS using starch mucilage, added towards the end of the titration, as indicator. At the same time carry out the operation in exactly the same manner, but without the substance being examined.
Calculate the iodine value from the expression 1.269 v/w where v is the difference, in mL, between the titrations and w is the weight, in g, of the substance taken. The approximate weight, in g, of the substance to be taken may be calculated by dividing 20 by the highest expected iodine value. If more than half of the available halogen is absorbed, the test must be repeated, using a smaller quantity of the substance.