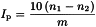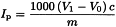Appendix X F. Peroxide Value
The peroxide value IP is the number that expresses in milliequivalents of active oxygen the quantity of peroxide contained in 1000 g of the substance, as determined by the methods described below.
When the monograph does not specify the method to be used, method A is applied. Any change from method A to method B is validated.
METHOD A
Place 5.00 g of the substance to be examined (m g) in a 250 mL conical flask fitted with a ground-glass stopper. Add 30 mL of a mixture of 2 volumes of chloroform R and 3 volumes of glacial acetic acid R. Shake to dissolve the substance and add 0.5 mL of saturated potassium iodide solution R. Shake for exactly 1 min then add 30 mL of water R. Titrate with 0.01 M sodium thiosulfate, adding the titrant slowly with continuous vigorous shaking, until the yellow colour is almost discharged. Add 5 mL of starch solution R and continue the titration, shaking vigorously, until the colour is discharged (n1 mL of 0.01 M sodium thiosulfate). Carry out a blank test under the same conditions (n2 mL of 0.01 M sodium thiosulfate). The volume of 0.01 M sodium thiosulfate used in the blank titration must not exceed 0.1 mL.
METHOD B
Carry out the operations avoiding exposure to actinic light.
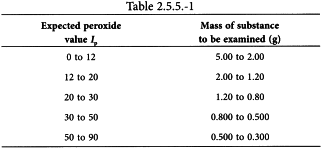
Place 50 mL of a mixture of 2 volumes of trimethylpentane R and 3 volumes of glacial acetic acid R in a conical flask and replace the stopper. Swirl the flask until the substance to be examined (m g; see Table 2.5.5.-1) has dissolved. Using a suitable volumetric pipette, add 0.5 mL of saturated potassium iodide solution R and replace the stopper. Allow the solution to stand for 60 ± 1 s, thoroughly shaking the solution continuously, then add 30 mL of water R.
Titrate the solution with 0.01 M sodium thiosulfate (V1 mL), adding it gradually and with constant, vigorous shaking, until the yellow iodine colour has almost disappeared. Add about 0.5 mL of starch solution R1 and continue the titration, with constant shaking especially near the end-point, to liberate all of the iodine from the solvent layer. Add the sodium thiosulfate solution dropwise until the blue colour just disappears.
Depending on the volume of 0.01 M sodium thiosulfate used, it may be necessary to titrate with 0.1 M sodium thiosulfate.
NOTE There is a 15 s to 30 s delay in neutralising the starch indicator for peroxide values of 70 and greater, due to the tendency of trimethylpentane to float on the surface of the aqueous medium and the time necessary to adequately mix the solvent and the aqueous titrant, thus liberating the last traces of iodine. It is recommended to use 0.1 M sodium thiosulfate for peroxide values greater than 150. A small amount (0.5 per cent to 1.0 per cent m/m) of high HLB emulsifier (for example polysorbate 60) may be added to the mixture to retard the phase separation and decrease the time lag in the liberation of iodine.
Carry out a blank determination (V0 mL). If the result of the blank determination exceeds 0.1 mL of titration reagent, replace the reagents and repeat the determination.
| c | = | concentration of the sodium thiosulfate solution, in moles per litre. |