Appendix XIV A. Microbiological Assay of Antibiotics
The potency of an antibiotic is estimated by comparing the inhibition of growth of sensitive micro-organisms produced by known concentrations of the antibiotic to be examined and a reference substance.
The reference substances used in the assays are substances whose activity has been precisely determined with reference to the corresponding international standard or international reference preparation.
The assay must be designed in a way that will permit examination of the validity of the mathematical model on which the potency equation is based. If a parallel-line model is chosen, the 2 log dose-response (or transformed response) lines of the preparation to be examined and the reference preparation must be parallel; they must be linear over the range of doses used in the calculation. These conditions must be verified by validity tests for a given probability, usually P = 0.05. Other mathematical models, such as the slope ratio model, may be used provided that proof of validity is demonstrated.
Unless otherwise stated in the monograph, the confidence limits (P = 0.95) of the assay for potency are not less than 95 per cent and not more than 105 per cent of the estimated potency.
Carry out the assay by method A or method B.
A. DIFFUSION METHOD
Liquefy a medium suitable for the conditions of the assay and inoculate it at a suitable temperature, for example 48 °C to 50 °C for vegetative forms, with a known quantity of a suspension of micro-organisms sensitive to the antibiotic to be examined, such that clearly defined zones of inhibition of suitable diameter are produced with the concentrations of the antibiotic used for the assay. Immediately pour into Petri dishes or large rectangular dishes a quantity of the inoculated medium to form a uniform layer 2-5 mm thick. Alternatively, the medium may consist of 2 layers, only the upper layer being inoculated.
Store the dishes so that no appreciable growth or death of the micro-organisms occurs before the dishes are used and so that the surface of the medium is dry at the time of use.
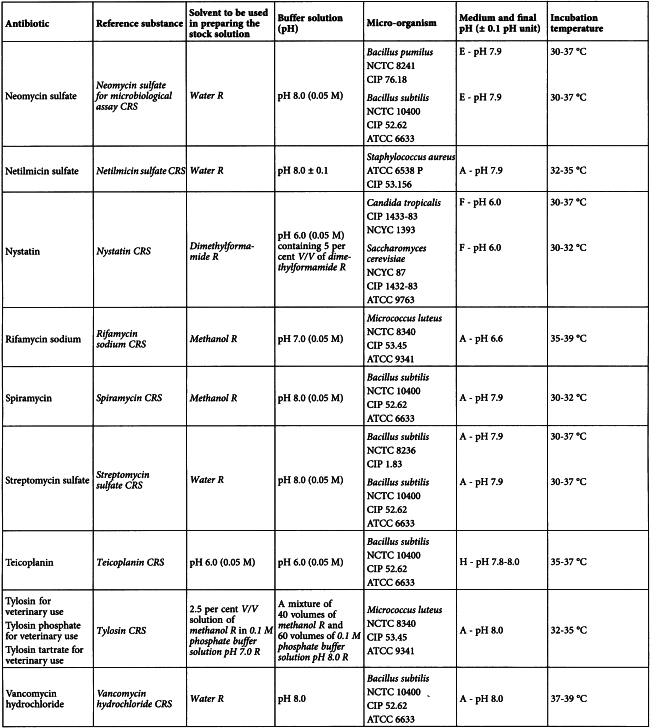
Using the solvent and the buffer solution indicated in Table 2.7.2.-1, prepare solutions of the reference substance and of the antibiotic to be examined having known concentrations and presumed to be of equal activity. Apply the solutions to the surface of the medium, for example, in sterile cylinders of porcelain, stainless steel or other suitable material, or in cavities prepared in the agar. The same volume of solution must be added to each cylinder or cavity. Alternatively, use sterile absorbent paper discs of suitable quality; impregnate the discs with the solutions of the reference substance or the solutions of the antibiotic to be examined and place on the surface of the agar.
In order to assess the validity of the assay, use not fewer than 3 doses of the reference substance and 3 doses of the antibiotic to be examined having the same presumed activity as the doses of the reference substance. It is preferable to use a series of doses in geometric progression. In routine assays when the linearity of the system has been demonstrated over an adequate number of experiments using a three-point assay, a two-point assay may be sufficient, subject to agreement by the competent authority. However, in all cases of dispute, a three-point assay as described above must be applied.
Arrange the solutions on each Petri dish or on each rectangular dish according to a statistically suitable design, except for small Petri dishes that cannot accommodate more than 6 solutions, arrange the solutions of the antibiotic to be examined and the solutions of the reference substance in an alternate manner to avoid interaction of the more concentrated solutions.
Incubate at a suitable temperature for about 18 h. A period of diffusion prior to incubation, usually 1-4 h, at room temperature or at about 4 °C, as appropriate, may be used to minimise the effects of the variation in time between the application of the solutions and to improve the regression slope.
Measure the diameters with a precision of at least 0.1 mm or the areas of the circular inhibition zones with a corresponding precision and calculate the potency using appropriate statistical methods.
Use in each assay the number of replications per dose sufficient to ensure the required precision. The assay may be repeated and the results combined statistically to obtain the required precision and to ascertain whether the potency of the antibiotic to be examined is not less than the minimum required.
B. TURBIDIMETRIC METHOD
Inoculate a suitable medium with a suspension of the chosen micro-organism having a sensitivity to the antibiotic to be examined such that a sufficiently large inhibition of microbial growth occurs in the conditions of the test. Use a known quantity of the suspension chosen so as to obtain a readily measurable opacity after an incubation period of about 4 h.
Use the inoculated medium immediately after its preparation.
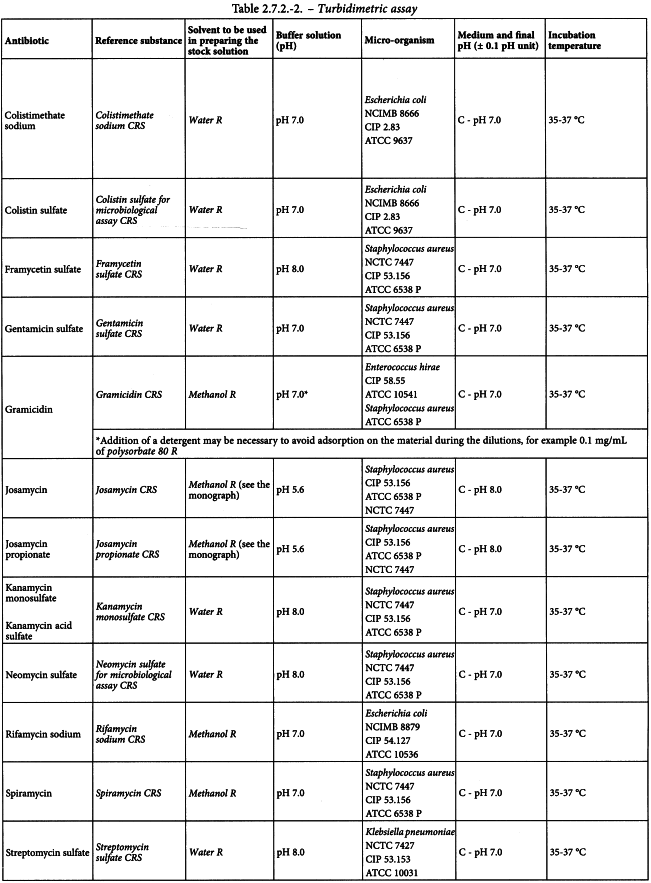
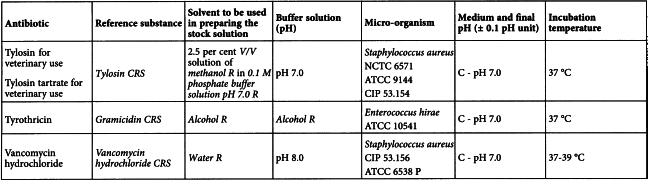
Using the solvent and the buffer solution indicated in Table 2.7.2.-2 prepare solutions of the reference substance and of the antibiotic to be examined having known concentrations presumed to be of equal activity.
In order that the validity of the assay may be assessed, use not fewer than 3 doses of the reference substance and 3 doses of the antibiotic to be examined having the same presumed activity as the doses of the reference substance. It is preferable to use a series of doses in geometric progression. In order to obtain the required linearity, it may be necessary to select from a large number 3 consecutive doses, using corresponding doses for the reference substance and the antibiotic to be examined.
Distribute an equal volume of each of the solutions into identical test-tubes and add to each tube an equal volume of inoculated medium (for example, 1 mL of the solution and 9 mL of the medium). For the assay of tyrothricin add 0.1 mL of the solution to 9.9 mL of inoculated medium.
Prepare at the same time 2 control tubes without antibiotic, both containing the inoculated medium and to one of which is added immediately 0.5 mL of formaldehyde R. These tubes are used to set the optical apparatus used to measure the growth.
Place all the tubes, randomly distributed or in a Latin square or randomised block arrangement, in a water-bath or other suitable apparatus fitted with a means of bringing all the tubes rapidly to the appropriate incubation temperature and maintain them at that temperature for 3-4 h, taking precautions to ensure uniformity of temperature and identical incubation time.
After incubation, stop the growth of the micro-organisms by adding 0.5 mL of formaldehyde R to each tube or by heat treatment and measure the opacity to 3 significant figures using suitable optical apparatus. Alternatively use a method which allows the opacity of each tube to be measured after exactly the same period of incubation.
Calculate the potency using appropriate statistical methods.
Linearity of the dose-response relationship, transformed or untransformed, is often obtained only over a very limited range. It is this range which must be used in calculating the activity and it must include at least 3 consecutive doses in order to permit linearity to be verified. In routine assays when the linearity of the system has been demonstrated over an adequate number of experiments using a three-point assay, a two-point assay may be sufficient, subject to agreement by the competent authority. However, in all cases of dispute, a three-point assay must be applied.
Use in each assay the number of replications per dose sufficient to ensure the required precision. The assay may be repeated and the results combined statistically to obtain the required precision and to ascertain whether the potency of the antibiotic to be examined is not less than the minimum required.
The following section is published for information.
Recommended micro-organisms
The following text details the recommended micro-organisms and the conditions of use. Other micro-organisms may be used provided that they are shown to be sensitive to the antibiotic to be examined and are used in appropriate media and appropriate conditions of temperature and pH. The concentrations of the solutions used should be chosen so as to ensure that a linear relationship exists between the logarithm of the dose and the response in the conditions of the test.
Preparation of inocula
Bacillus cereus var. mycoides; Bacillus subtilis; Bacillus pumilus. Spore suspensions of the organisms to be used as inocula are prepared as follows.
Grow the organism at 35-37 °C for 7 days on the surface of a suitable medium to which has been added 0.001 g/L of manganese sulfate R. Using sterile water R, wash off the growth, which consists mainly of spores. Heat the suspension at 70 °C for 30 min and dilute to give an appropriate concentration of spores, usually 10 × 106 to 100 × 106 per millilitre. The spore suspensions may be stored for long periods at a temperature not exceeding 4 °C.
Alternatively, spore suspensions may be prepared by cultivating the organisms in medium C at 26 °C for 4-6 days, then adding, aseptically, sufficient manganese sulfate R to give a concentration of 0.001 g/L and incubating for a further 48 h. Examine the suspension microscopically to ensure that adequate spore formation has taken place (about 80 per cent) and centrifuge. Re-suspend the sediment in sterile water R to give a concentration of 10 × 106 to 100 × 106 spores per millilitre, and then heat to 70 °C for 30 min. Store the suspension at a temperature not exceeding 4 °C.
Bordetella bronchiseptica Grow the test organism on medium B at 35-37 °C for 16-18 h. Wash off the bacterial growth with sterile water R and dilute to a suitable opacity.
Staphylococcus aureus; Klebsiella pneumoniae; Escherichia coli; Micrococcus luteus; Staphylococcus epidermidis. Prepare as described above for B. bronchiseptica but using medium A and adjusting the opacity to one which has been shown to produce a satisfactory dose-response relationship in the turbidimetric assay, or to produce clearly defined zones of inhibition of convenient diameter in the diffusion assay, as appropriate.
Saccharomyces cerevisiae; Candida tropicalis. Grow the test organism on medium F at 30-37 °C for 24 h. Wash off the growth with a sterile 9 g/L solution of sodium chloride R. Dilute to a suitable opacity with the same solution.
Buffer solutions
Buffer solutions having a pH between 5.8 and 8.0 are prepared by mixing 50.0 mL of 0.2 M potassium dihydrogen phosphate R with the quantity of 0.2 M sodium hydroxide indicated in Table 2.7.2.-3. Dilute with freshly prepared distilled water R to produce 200.0 mL.
These buffer solutions are used for all microbiological assays shown in Table 2.7.2.-1 with the exception of bleomycin sulfate and amphotericin B.
For bleomycin sulfate, prepare the buffer solution pH 6.8 as follows: dissolve 6.4 g of potassium dihydrogen phosphate R and 18.9 g of disodium hydrogen phosphate dodecahydrate R in water R and dilute to 1000 mL with water R.
For amphotericin B, prepare the 0.2 M phosphate buffer solution pH 10.5 as follows: dissolve 35 g of dipotassium hydrogen phosphate R in 900 mL of water R, add 20 mL of 1 M sodium hydroxide and dilute to 1000.0 mL with water R.
Culture media
The following media or equivalent media may be used.
Medium A | ||
|---|---|---|
Peptone | 6 g | |
Pancreatic digest of casein | 4 g | |
Beef extract | 1.5 g | |
Yeast extract | 3 g | |
Glucose monohydrate | 1 g | |
Agar | 15 g | |
Water | to 1000 mL | |
Medium B | ||
|---|---|---|
Pancreatic digest of casein | 17 g | |
Papaic digest of soya bean | 3 g | |
Sodium chloride | 5 g | |
Dipotassium hydrogen phosphate | 2.5 g | |
Glucose monohydrate | 2.5 g | |
Agar | 15 g | |
Polysorbate 80 | 10 g | |
Water | to 1000 mL | |
The polysorbate 80 is added to the hot solution of the other ingredients after boiling, and immediately before adjusting to volume.
Medium C | ||
|---|---|---|
Peptone | 6 g | |
Beef extract | 1.5 g | |
Yeast extract | 3 g | |
Sodium chloride | 3.5 g | |
Glucose monohydrate | 1 g | |
Dipotassium hydrogen phosphate | 3.68 g | |
Potassium dihydrogen phosphate | 1.32 g | |
Water | to 1000 mL | |
Medium D | ||
|---|---|---|
Heart extract | 1.5 g | |
Yeast extract | 1.5 g | |
Peptone-casein | 5 g | |
Glucose monohydrate | 1 g | |
Sodium chloride | 3.5 g | |
Dipotassium hydrogen phosphate | 3.68 g | |
Potassium dihydrogen phosphate | 1.32 g | |
Potassium nitrate | 2 g | |
Water | to 1000 mL | |
Medium E | ||
|---|---|---|
Peptone | 5 g | |
Meat extract | 3 g | |
Disodium hydrogen phosphate,12H2O | 26.9 g | |
Agar | 10 g | |
Water | to 1000 mL | |
The disodium hydrogen phosphate is added as a sterile solution after sterilisation of the medium.
Medium F | ||
|---|---|---|
Peptone | 9.4 g | |
Yeast extract | 4.7 g | |
Beef extract | 2.4 g | |
Sodium chloride | 10.0 g | |
Glucose monohydrate | 10.0 g | |
Agar | 23.5 g | |
Water | to 1000 mL | |
Medium G | ||
|---|---|---|
Glycerol | 10 g | |
Peptone | 10 g | |
Meat extract | 10 g | |
Sodium chloride | 3 g | |
Agar | 15 g | |
Water | to 1000 mL | |
pH 7.0 ± 0.1 after sterilisation.
Medium H | ||
|---|---|---|
Peptone | 5.0 g | |
Agar | 15.0 g | |
Beef extract powder | 3.0 g | |
Water | to 1000 mL | |
pH 7.8 - 8.0 adjusted with 0.1 M sodium hydroxide.