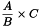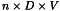Appendix XIV N1. Numeration of CD34/CD45+ Cells in Haematopoietic Products
This chapter describes immunolabelling and analysis by flow cytometry (2.7.24) to determine the number of CD34/CD45+ cells contained in haematopoietic products. The determination is carried out by a single platform method using calibrated fluorospheres, after lysis of the sample red blood cells if necessary.
This method applies to all types of preparations and whole blood. However, its level of precision makes it particularly suitable for preparations containing very low percentages of CD34/CD45+ cells.
Graft quality assessment by CD34/CD45+ cell enumeration
A variety of studies have established that the 1-3 per cent of cells in the bone marrow that express the CD34 cell surface antigen are capable of reconstituting long-term, multilineage haematopoiesis after myeloablative therapy. CD34/CD45+ cells are also found in the peripheral circulation of normal individuals but are extremely rare (0.01-0.1 per cent). However, CD34/CD45+ cells may also be mobilised from marrow to the peripheral circulation in greater numbers by haematopoietic cytokines such as granulocyte colony-stimulating factor and/or chemotherapy.
The technique used for enumeration of CD34/CD45+ cells must meet the following requirements:
Selection of parameters
The flow cytometry assay uses commercially available, directly conjugated fluorochrome-labelled monoclonal antibodies, routine staining and whole blood lysing procedures, and a gating strategy using light scatter and immunofluorescence analysis using a pan-CD45/CD34 monoclonal antibody combination.
It is possible to determine CD34/CD45+ cell viability by appropriate nucleic acid staining with a stain that does not cross the intact cell membrane (for example, with 7-aminoactinomycin D).
Selection of monoclonal antibodies
CD34 antibodies Use class III CD34 antibodies that detect all glycosylation variants of the molecule (for example, clone 8G12 or 581). To detect rare events, use an antibody conjugated to the brightest fluorochrome excitable using an argon laser-based flow cytometer, for example phycoerythrin (PE).
CD45 antibodies Pan-CD45 antibodies that detect all isoforms and all glycoforms of this structure are required. A CD45 antibody conjugated to fluorescein isothiocyanate (FITC) fluorochrome is generally used (for example, J33, HLe1, 2D1).
Isotypic or isoclonic controls A negative control is analysed to detect any non-specific signal in the PE fluorescence region. If using an isotypic control (a monoclonal antibody to an irrelevant antigen of the same isotype as the CD34 antibody employed), the PE-conjugated isotype is combined with CD45-FITC (or PerCP). If using an isoclonic control, the unconjugated (in excess) and PE-conjugated CD34 identical monoclonal antibody is combined with conjugated CD45. Alternative combinations may be used.
Absolute count of CD34/CD45+
Calibrated fluorospheres Depending on the technique used, the internal standard either consists of calibrated beads in suspension or is directly introduced into the associated tubes by the manufacturer.
The absolute count of the CD34/CD45+ cells per microlitre is calculated using the following expression:
| A | = | number of CD34/CD45+ cells counted; |
| B | = | number of fluorosphere singlets counted; |
| C | = | known fluorosphere concentration. |
Gating strategies
The purpose of sequential gating is to select the population of interest and simultaneously minimise interference from debris and mature cells to which antibodies can bind non-specifically. If using a commercial kit, apply the gating recommended by the manufacturer. If using an in-house assay, it is preferable to apply a currently recommended strategy. A gating strategy that uses light scattering parameters and CD34/CD45 fluorescence will aid in the accurate identification and enumeration of CD34/CD45+ cells.
Number of events analysed
A sufficient number of events are analysed to maintain acceptable precision, for example not fewer than 100 CD34+ events and not fewer than 60 000 CD45+ events; the total number of cells counted may be greater if the percentage of CD34 is 0.1 per cent or less.
Specimen collection
Acid citrate dextrose (ACD) formula A is the anticoagulant used in apheresis procedures. This anticoagulant allows both an automated leucocyte count and flow cytometry evaluation to be performed on the same specimen. Edetic acid (EDTA) is the anticoagulant of choice for peripheral blood sampling.
Specimen transport
Transport conditions guarantee the physical and thermal safety of samples.
Specimen integrity and storage
Fresh (less than 24 h old) apheresis products, whole blood samples, umbilical cord blood specimens or bone marrow samples can be processed. Old specimens (more than 24 h old) and specimens that have been frozen and thawed are stained with a viability dye. On receipt, the temperature within the package is verified.
TECHNIQUE
Sample preparation
Ensure that the concentration of leucocytes is suitable prior to staining with monoclonal antibodies. If necessary, dilute the sample with medium that is compatible with the product to be tested and the lysing system. Record the dilution factor. It is recommended to perform the test with a negative control.
Flow cytometry analysis
Autostandardisation
For analysis of cells labelled with a commercially available kit, manufacturers have developed some quality tools for setting the flow cytometer. These settings are then automatically transferred on protocol analysis of samples. Specific fluorospheres are used to set the photomultiplier tube (PMT) on target values, compensation is set and the system is checked using a control preparation.
System settings
Calculation of absolute number of CD34/CD45+ cells
The absolute number of CD34/CD45+ cells is calculated using the following expression:
| n | = | total number of CD34/CD45+ cells per microlitre; |
| D | = | dilution factor; |
| V | = | volume of the product to be tested, in microlitres. |
Results are reported as both the percentage of CD34/CD45+ cells and the absolute number per microlitre. They may also be reported as the absolute number per kilogram of recipient body mass, where this is possible.