Appendix XIV P. Determination of Bactericidal, Fungicidal or Yeasticidal Activity of Antiseptic Medicinal Products
This general chapter describes a test that can be used for the determination of antimicrobial activity in antiseptic medicinal products that are miscible with water and intended for administration by direct contact with the skin or mucous membranes. The extent of testing is dependent on the declared antimicrobial activity of the product.
The test determines whether a product exhibits bactericidal, fungicidal or yeasticidal activity and complies with an established specification for such activity.
This test cannot replace or confirm the assessment of the clinical efficacy of such preparations.
1 PRINCIPLE
Antimicrobial activity is determined by adding test suspensions of micro-organisms (bacteria, fungi or yeasts, separately) to the sample antiseptic product. The mixture is maintained at 33 ± 1 °C for contact times of 5 min for bactericidal activity and 15 min for fungicidal or yeasticidal activity. Additional contact times may be chosen, according to the stated use of the antiseptic medicinal product. At the end of the contact time, an aliquot is taken and the antimicrobial activity in this aliquot is immediately stopped by a validated method. 2 methods are available: dilution-neutralisation and membrane filtration.
The procedure is validated to verify its ability to demonstrate the required reduction in the count of viable micro-organisms by the use of appropriate controls.
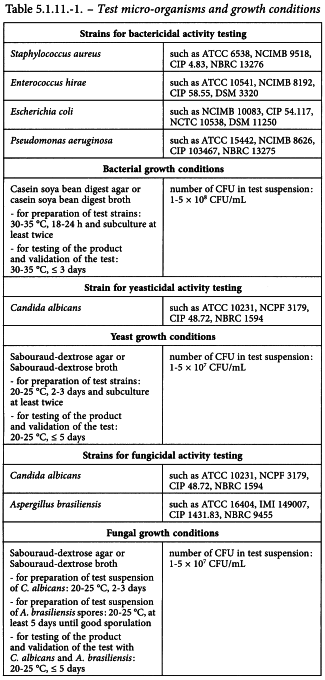
2 TEST MICRO-ORGANISMS AND GROWTH CONDITIONS
Prepare standardised stable suspensions of test strains as stated in section 2-1. Seed-lot culture maintenance techniques (seed-lot systems) are used so that the viable micro-organisms used for inoculation are not more than 5 passages removed from the original master seed-lot. Grow each of the microbial test strains separately as described in Table 5.1.11.-1.
The recommended solutions and media are described in general chapter 2.6.13. Purified water is used. All reagents are sterile prior to use.
The test for bactericidal, fungicidal or yeasticidal activity is performed with the designated strains as described in Table 5.1.11.-1. In addition to these micro-organisms, it may be necessary to add other bacterial or fungal strains that represent the indications of the antiseptic medicinal product tested.
Single-strain challenges are used. The counts are performed in duplicate and the arithmetic mean of the results is calculated and expressed in CFU/mL.
2-1 PREPARATION OF TEST SUSPENSION
For harvesting the micro-organisms use a sufficient volume of a 9 g/L solution of sodium chloride R (for bacteria and C. albicans) or a solution containing 9 g/L of sodium chloride R and 0.5 g/L of polysorbate 80 R (for A. brasiliensis), to obtain a test suspension with the number of CFU described in Table 5.1.11.-1. Use the suspension within 2 h or within 24 h if stored at 2-8 °C.
2-2 PREPARATION OF ANTISEPTIC PRODUCT TEST SOLUTION
The concentration of the antiseptic product test solution shall be, if possible, 1.25 times the in-use test concentration because it is diluted to 80 per cent during the test and the method validation.
2-3 NEUTRALISING AGENTS
Neutralising agents are used to neutralise the antimicrobial activity of the antiseptic product. The common neutralising agents are listed in Table 2.6.12.-2 of general chapter 2.6.12. Microbiological examination of non-sterile products: microbial enumeration tests. The neutralisation time is not less than 10 s and not more than 60 s.
3 METHODS
Prior to testing, equilibrate the temperature of all reagents to 33 ± 1 °C.
3-1 DILUTION-NEUTRALISATION METHOD
Transfer 1.0 mL of a 3 g/L solution of bovine albumin R into a tube, add 1.0 mL of the test suspension and maintain at 33 ± 1 °C for 2 min. Add 8.0 mL of the antiseptic product test solution and maintain at 33 ± 1 °C for the chosen contact time. Then, take a 1.0 mL sample of the test mixture and transfer into a tube containing 1.0 mL of water R and 8.0 mL of the neutralising agent and maintain at 33 ± 1 °C for the appropriate neutralisation time. Take 1.0 mL of the neutralised test mixture, in duplicate, and inoculate using the pour-plate or surface-spread method. For incubation conditions, see Table 5.1.11.-1. After incubation, perform the count.
3-1-1 Suitability of the test/controls
For all methods, prepare a validation suspension containing 100-1000 CFU of the test micro-organisms per millilitre.
3-1-1-1Experimental conditions control
Transfer 1.0 mL of a 3 g/L solution of bovine albumin R into a tube, add 1.0 mL of the validation suspension and maintain at 33 ± 1 °C for 2 min. Add 8.0 mL of water R and maintain at 33 ± 1 °C for the chosen contact time. Take 1.0 mL of this mixture, in duplicate, and inoculate using the pour-plate or surface-spread method. For incubation conditions, see Table 5.1.11.-1. After incubation, perform the count. The number of CFU recovered following incubation is not less than 0.5 × (number of CFU in the validation suspension)/10.
3-1-1-2Neutralising agent control
Transfer 1.0 mL of a 3 g/L solution of bovine albumin R into a tube, add 1.0 mL of the validation suspension and 8.0 mL of the neutralising agent used in the test and maintain at 33 ± 1 °C for the appropriate neutralisation time. Take 1.0 mL of this mixture, in duplicate, and inoculate using the pour-plate or surface-spread method. For incubation conditions, see Table 5.1.11.-1. After incubation, perform the count. The number of CFU recovered following incubation is not less than 0.5 × (number of CFU in the validation suspension)/10.
3-1-1-3Dilution-neutralisation method control
Transfer 1.0 mL of a 3 g/L solution of bovine albumin R into a tube, add 1.0 mL of a 9 g/L solution of sodium chloride R and 8.0 mL of the product test solution and maintain at 33 ± 1 °C for the chosen contact time. Transfer 1.0 mL of this mixture into a tube containing 8.0 mL of the neutralising agent and maintain at 33 ± 1 °C for the appropriate neutralisation time. Then add 1.0 mL of the validation suspension and mix. After 30 min, take a sample of 1.0 mL of the mixture, in duplicate, and inoculate using the pour-plate or surface-spread method. For incubation conditions, see Table 5.1.11.-1. After incubation, perform the count. The number of CFU recovered following incubation is not less than 0.5 × (number of CFU in the validation suspension)/10.
3-2 MEMBRANE FILTRATION METHOD
Proceed as described in section 3-1, carrying out immediately the filtration step in place of the neutralisation step.
Use membrane filters having a nominal pore size not greater than 0.45 µm. The type of filter material is chosen such that the microbe-retaining efficiency is not affected by the components of the sample to be investigated. For each of the micro-organisms listed, a single membrane filter is used. Appropriately dilute 0.1 mL of the test solution and immediately filter the total volume, then rinse the membrane filter with an appropriate volume of the diluent. Perform the test in duplicate. For incubation conditions, see Table 5.1.11.-1. After incubation, perform the count.
3-2-1 Verification of the selected experimental conditions and of the membrane filtration method
3-2-1-1Experimental conditions control
Proceed as described in section 3-1-1-1, except at the end of the contact time, take the sample in duplicate, and transfer into a separate membrane filtration apparatus. Filter immediately and then transfer each of the membrane filters to the surface of separate plates. For incubation conditions, see Table 5.1.11.-1. After incubation, perform the count. The number of CFU recovered following incubation is not less than 0.5 × (number of CFU in the validation suspension)/10.
3-2-1-2Membrane filtration method control
Proceed as described in section 3-1-1-3, except at the end of the chosen contact time, take that sample in duplicate, and transfer into a separate membrane filtration apparatus. Filter and rinse as described in section 3-2, then cover the membranes with rinsing liquid and add a sample of the validation suspension. Filter again and transfer each of the membrane filters to the surface of separate plates. For incubation conditions, see Table 5.1.11.-1. After incubation, perform the count. The number of CFU recovered following incubation is not less than 0.5 × (number of CFU in the validation suspension)/10.
4 ACCEPTANCE CRITERIA
Unless otherwise justified and authorised, the preparation has a: