Appendix XVI C. Efficacy of Antimicrobial Preservation
If a pharmaceutical preparation does not itself have adequate antimicrobial activity, antimicrobial preservatives may be added, particularly to aqueous preparations, to prevent proliferation or to limit microbial contamination which, during normal conditions of storage and use, particularly for multidose containers, could occur in a product and present a hazard to the patient from infection and spoilage of the preparation. Antimicrobial preservatives must not be used as a substitute for good manufacturing practice.
The efficacy of an antimicrobial preservative may be enhanced or diminished by the active constituent of the preparation or by the formulation in which it is incorporated or by the container and closure used. The antimicrobial activity of the preparation in its final container is investigated over the period of validity to ensure that such activity has not been impaired by storage. Such investigations may be carried out on samples removed from the final container immediately prior to testing.
During development of a pharmaceutical preparation, it shall be demonstrated that the antimicrobial activity of the preparation as such or, if necessary, with the addition of a suitable preservative or preservatives provides adequate protection from adverse effects that may arise from microbial contamination or proliferation during storage and use of the preparation.
The efficacy of the antimicrobial activity may be demonstrated by the test described below. The test is not intended to be used for routine control purposes.
TEST FOR EFFICACY OF ANTIMICROBIAL PRESERVATION
The test consists of challenging the preparation, wherever possible in its final container, with a prescribed inoculum of suitable micro-organisms, storing the inoculated preparation at a prescribed temperature, withdrawing samples from the container at specified intervals of time and counting the organisms in the samples so removed.
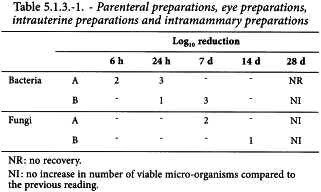
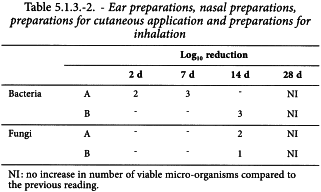
The preservative properties of the preparation are adequate if, in the conditions of the test, there is a significant fall or no increase, as appropriate, in the number of micro-organisms in the inoculated preparation after the times and at the temperatures prescribed. The acceptance criteria, in terms of decrease in the number of micro-organisms with time, vary for different types of preparations according to the degree of protection intended (see Tables 5.1.3.-1/2/3).
Single-strain challenges are used and the designated micro-organisms are supplemented, where appropriate, by other strains or species that may represent likely contaminants to the preparation. It is recommended, for example, that Escherichia coli (ATCC 8739; NCIMB 8545; CIP 53.126) is used for all oral preparations and Zygosaccharomyces rouxii (NCYC 381; IP 2021.92) for oral preparations containing a high concentration of sugar.
Preparation of inoculum
Preparatory to the test, inoculate the surface of casein soya bean digest agar (2.6.12) for bacteria or Sabouraud-dextrose agar without the addition of antibiotics (2.6.12) for fungi, with the recently grown stock culture of each of the specified micro-organisms. Incubate the bacterial cultures at 30-35 °C for 18-24 h, the culture of C. albicans at 20-25 °C for 48 h, and the culture of A. brasiliensis at 20-25 °C for 1 week or until good sporulation is obtained. Subcultures may be needed after revival before the micro-organism is in its optimal state, but it is recommended that their number be kept to a minimum.
To harvest the bacterial and C. albicans cultures, use a sterile suspending fluid, containing 9 g/L of sodium chloride R, for dispersal and transfer of the surface growth into a suitable vessel. Add sufficient suspending fluid to reduce the microbial count to about 108 micro-organisms per millilitre. To harvest the A. brasiliensis culture, use a sterile suspending fluid containing 9 g/L of sodium chloride R and 0.5 g/L of polysorbate 80 R and adjust the spore count to about 108 per millilitre by adding the same solution.
Remove immediately a suitable sample from each suspension and determine the number of colony-forming units per millilitre in each suspension by plate count or membrane filtration (2.6.12). This value serves to determine the inoculum and the baseline to use in the test. The suspensions shall be used immediately.
METHOD
To count the viable micro-organisms in the inoculated products, use the agar medium used for the initial cultivation of the respective micro-organisms.
Inoculate a series of containers of the product to be examined, each with a suspension of one of the test organisms to give an inoculum of 105 to 106 micro-organisms per millilitre or per gram of the preparation. The volume of the suspension of inoculum does not exceed 1 per cent of the volume of the product. Mix thoroughly to ensure homogeneous distribution.
Maintain the inoculated product at 20-25 °C, protected from light. Remove a suitable sample from each container, typically 1 mL or 1 g, at zero hour and at appropriate intervals according to the type of the product and determine the number of viable micro-organisms by plate count or membrane filtration (2.6.12). Ensure that any residual antimicrobial activity of the product is eliminated by dilution, by filtration or by the use of a specific inactivator. When dilution procedures are used, due allowance is made for the reduced sensitivity in the recovery of small numbers of viable micro-organisms. When a specific inactivator is used, the ability of the system to support the growth of the test organisms is confirmed by the use of appropriate controls.
The procedure is validated to verify its ability to demonstrate the required reduction in count of viable micro-organisms.
ACCEPTANCE CRITERIA
The criteria for evaluation of antimicrobial activity are given in Tables 5.1.3.-1/2/3 in terms of the log10 reduction in the number of viable micro-organisms against the value obtained for the inoculum.
The A criteria express the recommended efficacy to be achieved. In justified cases where the A criteria cannot be attained, for example for reasons of an increased risk of adverse reactions, the B criteria must be satisfied.
The A criteria express the recommended efficacy to be achieved. In justified cases where the A criteria cannot be attained, for example for reasons of an increased risk of adverse reactions, the B criteria must be satisfied.
The above criteria express the recommended efficacy to be achieved.