Appendix XXII B. Minimising the Risk of Transmitting Animal Spongiform Encephalopathy Agents Via Human and Veterinary Medicinal Products
Contents
1. INTRODUCTION
1-1. Scientific background
1-2. Regulatory compliance
2. SCOPE
3. GENERAL CONSIDERATIONS
3-1. Scientific principles for minimising risk
3-2. Animal source
3-2-1. Geographical sourcing
3-2-1-1. Bovine materials
3-2-1-2. Sheep and goats (small ruminants)
3-2-2. BSE negligible risk (closed) bovine herds
3-3. Animal parts, body fluids and secretions as starting material
3-4. Age of animals
3-5. Manufacturing Process
4. RISK ASSESSMENT OF MATERIALS OR SUBSTANCES USED IN THE MANUFACTURE AND PREPARATION OF A MEDICINAL PRODUCT IN THE CONTEXT OF REGULATORY COMPLIANCE
5. BENEFIT/RISK EVALUATION
6. SPECIFIC CONSIDERATIONS
6-1. Collagen
6-2. Gelatin
6-3. Bovine blood and blood derivatives
6-4. Tallow derivatives
6-5. Animal charcoal
6-6. Milk and milk derivatives
6-7. Wool derivatives
6-8. Amino acids
6-9. Peptones
1 Introduction
1-1 Scientific background
Transmissible Spongiform Encephalopathies (TSEs) are chronic degenerative nervous diseases characterised by the accumulation of an abnormal isoform of a cellular glycoprotein (known as PrP or prion protein). The abnormal isoform of PrP (PrPTSE) differs from normal PrP (PrPc) in being highly resistant to protease and heat denaturation treatments. PrPTSE is considered to be the infective agent responsible for transmitting TSE disease.
TSE diseases in animals include:
In humans, spongiform encephalopathies include different forms of Creutzfeldt-Jakob Disease (CJD), Kuru, Gerstmann-Sträussler-Scheinker Syndrome (GSS), and Fatal Familial Insomnia (FFI).
Iatrogenic transmission of spongiform encephalopathies has been reported. In sheep, scrapie has been accidentally transmitted by the use of Louping Ill vaccine prepared from pooled, formaldehyde treated ovine brain and spleen in which material from scrapie-infected sheep had been inadvertently incorporated. Also, transmission of scrapie to sheep and goats occurred following use of a formol-inactivated vaccine against contagious agalactia, prepared with brain and mammary gland homogenates of sheep infected with Mycoplasma agalactiae. In man, cases of transmission of CJD have been reported which have been attributed to the parenteral administration of growth hormone and gonadotropin derived from human cadaveric pituitary glands. Cases of CJD have also been attributed to the use of contaminated instruments in brain surgery and with the transplantation of human dura mater and cornea.
Interspecies TSE transmission is restricted by a number of natural barriers, transmissibility being affected by the species of origin, the prion strain, dose, route of exposure and, in some species, the host allele of the PRNP gene. Species barriers can be crossed under appropriate conditions.
BSE was first diagnosed in the United Kingdom in 1986 and a large number of cattle and individual herds have been affected. It is clear that BSE is a food borne disease associated with feed (e.g. meat and bone meal) derived from TSE affected animals. Other countries have experienced cases of BSE, either in animals imported from the United Kingdom or in indigenous animals. There is convincing evidence to show that the variant form of CJD (vCJD) is caused by the agent which is responsible for BSE in cattle. Therefore, a cautious approach continues to be warranted if biological materials from species naturally affected by TSE diseases, especially bovine species, are used for the manufacture of medicinal products.
In the course of active surveillance programs, two previously unrecognized forms of atypical BSE (BSE-L, also named BASE, and BSE-H) have been identified in rare sporadic cases from Europe, North America, and Japan. The ‘L’ and ‘H’ identify the higher and lower electrophoretic positions of their protease-resistant PrPTSE isoforms. It is noteworthy that atypical cases have been found in countries that did not experience classical BSE so far, like Sweden, or in which only few classical BSE cases have been found like Canada or USA. The atypical BSE agent has been experimentally transmitted to transgenic mice expressing the human prion protein and to a cynomolgus monkey.
Scrapie occurs worldwide and has been reported in most European countries. It has the highest incidence in Cyprus. While humans have been exposed to naturally occurring scrapie for over 250 years, there is no epidemiological evidence directly linking scrapie to spongiform encephalopathies in humans1 . However, there remains a theoretical and currently unquantifiable risk that some BSE-contaminated protein supplement may have been fed to sheep. Further, it should also be assumed that any BSE agent introduced into the small ruminant population via contaminated feed is likely to be recycled and amplified2 .
There is interest in infecting cells with TSE agents to develop assays and for basic scientific reasons. Some success has been reported, usually but not always with neural cell lines. The conditions needed to infect a cell are not well understood and the process is difficult requiring particular combinations of agent and cell. It is not considered appropriate to make specific recommendations in terms of cell substrates to be used for production of biological/biotechnology-derived substances. Nevertheless, the possibility of infection of cell lines with TSE agents should be taken into account in risk assessments.
1-2 Regulatory compliance
Risk assessment
Since the use of animal-derived materials is unavoidable for the production of some medicinal products and that complete elimination of risk at source is rarely possible, the measures taken to manage the risk of transmitting animal TSEs via medicinal products represent risk minimisation rather than risk elimination. Consequently, the basis for regulatory compliance should be based on a risk assessment, taking into consideration all pertinent factors as identified in this chapter (see below).
Legal basis
The note for guidance is published by the European Commission following
These directives require that applicants for marketing authorisation for human and veterinary medicinal products must demonstrate that medicinal products are manufactured in accordance with the latest version of this note for guidance published in the Official Journal of the European Union. This is a continuing obligation after the marketing authorisation has been granted.
By definition, the principle of Specified Risk Materials as defined in Regulation (EC) No 999/2001 of the European Parliament and of the Council5 does not apply to medicinal products. However, Regulation (EC) No 1774/2002 of the European Parliament and of the Council6 , which applies since 1st May 2003, lays down health rules concerning animal by-products not intended for human consumption. As a general rule, and unless properly justified, all animal by-products used as starting materials in the manufacture of medicinal products should be ‘Category 3 (i.e. safe) materials or equivalent’, as defined in Regulation (EC) No 1774/2002. Justification for the use of substances derived from other, high infectivity materials must follow an appropriate benefit/risk evaluation (see further below).
The note for guidance should be read in conjunction with the various EU legal instruments including Commission decisions progressively implemented since 1991. Where appropriate, references to these decisions are given in the text. Position statements and explanatory notes made by the Committee for Medicinal Products for Human Use (CHMP) and Committee for Medicinal Products for Veterinary Use (CVMP) are still applicable for the purpose of regulatory compliance unless otherwise superseded by the note for guidance.
The general monograph Products with risk of transmitting agents of animal spongiform encephalopathies of the European Pharmacopoeia refers to this chapter, which is identical with the note for guidance. The monograph forms the basis for issuing Certificates of Suitability as a procedure for demonstrating TSE compliance for substances and materials used in the manufacture of human and veterinary medicinal products.
Clarification of note for guidance
As the scientific understanding of TSEs, especially the pathogenesis of the diseases, is evolving, from time to time CHMP and its Biologics Working Party in collaboration with CVMP and its Immunologicals Working Party may be required in the future to develop supplementary guidance in the form of position statements or explanatory notes for the purpose of clarifying the note for guidance. The supplementary guidance shall be published by the Commission and on the website of the European Medicines Agency and taken into consideration accordingly in the scope of the certification of the European Directorate for the Quality of Medicines & HealthCare (EDQM).
2 Scope
TSE-relevant animal species
Cattle, sheep, goats and animals that are naturally susceptible to infection with transmissible spongiform encephalopathy agents or susceptible to infection through the oral route other than humans7 and non-human primates are defined as “TSE-relevant animal species” 8 .
Materials
This chapter is concerned with materials derived from “TSE-relevant animal species” that are used for the preparation of:
This chapter is also applicable to materials that come into direct contact with the equipment used in manufacture of the medicinal product or that come in contact with the medicinal product and therefore have the potential for contamination.
Materials used in the qualification of plant and equipment, such as culture media used in media fill experiments to validate the aseptic filling process, shall be considered in compliance with this chapter provided that the constituent or constituents are derived from tissues with no detectable infectivity (category IC tissues), where the risk of cross-contamination with potentially infective tissues has been considered (see section 3-3) and where the materials are sourced from countries with negligible BSE risk or controlled BSE risk (Categories A and B, respectively – see section 3-2). Such information shall be provided in the dossier for a marketing authorisation and verified during routine inspection for compliance with Good Manufacturing Practice (GMP).
Other materials such as cleaning agents, softeners and lubricants that come into contact with the medicinal product during its routine manufacture or in the finishing stage or in the primary packaging are considered in compliance with this chapter if they are tallow derivatives prepared using the rigorous physicochemical processes as described in section 6.
Seed lots, cell banks and routine fermentation/production9
For the purpose of regulatory compliance, master seeds or master cell banks in marketing authorisation applications lodged after 1 July 2000 (for human medicinal products) or 1 October 2000 (for veterinary medicinal products) shall be covered by the note for guidance.
Master seeds and master cell banks,
that have already been approved for the manufacture of a constituent of an authorised medicinal product shall be considered in compliance with the note for guidance even if they are incorporated in marketing authorisation applications lodged after 1 July 2000 (for human medicinal products) or 1 October 2000 (for veterinary medicinal products).
Master cell banks and master seeds established before 1 July 2000 (for human medicinal products) or 1 October 2000 (for veterinary medicinal products), but not yet approved as a constituent of an authorised medicinal product shall demonstrate that they fulfil the requirements of the note for guidance. If, for some raw or starting materials or reagents used for the establishment of these cell banks or seeds, full documentary evidence is no longer available, the applicant should present a risk assessment as described in Section 4 of the note for guidance.
Established working seeds or cell banks used in the manufacture of medicinal products authorised before 1 July 2000 (human medicines) or 1 October 2000 (veterinary medicines), which have been subjected to a properly conducted risk assessment by a Competent Authority of the Member States or the European Medicines Agency and declared to be acceptable, shall also be considered compliant.
However, where materials derived from the “TSE-relevant animal species” are used in fermentation/routine production processes or in the establishment of working seeds and working cell banks, the applicant must demonstrate that they fulfil the requirements of the note for guidance.
3 General considerations
3-1 Scientific principles for minimising risk
When manufacturers have a choice, the use of materials from “non TSE-relevant animal species” or non-animal origin is preferred. The rationale for using materials derived from “TSE-relevant animal species” instead of materials from “non-TSE-relevant species” or of non-animal origin should be given. If materials from “TSE-relevant animal species” have to be used, consideration should be given to all the necessary measures to minimise the risk of transmission of TSE.
Readily applicable diagnostic tests for TSE infectivity in vivo are not yet available. Diagnosis is based on post-mortem confirmation of characteristic brain lesions by histopathology and/or detection of PrPTSE by Western blot or immunoassay. The demonstration of infectivity by the inoculation of suspect tissue into target species or laboratory animals is also used for confirmation. However, due to the long incubation periods of all TSEs, results of in vivo tests are available only after months or years.
Several immunochemical tests have been developed for the detection of PrPTSE in post-mortem samples and some are now considered to be extremely sensitive. However, their ability to detect an infected animal depends on the timing of sample collection in relation to timing of exposure, the type of tissue collected and infectious dose acquired, together with consequential timing of onset of clinical disease. There is currently insufficient information on how this might be affected by strain variations.
Although screening of source animals by in vitro tests may prevent the use of animals at late stages of incubation of the disease and may provide information about the epidemiological status of a given country or region, none of the tests are considered suitable to unambiguously confirm the negative status of an animal.
Minimising the risks of transmission of TSE is based upon three complementary parameters:
3-2 ANIMAL SOURCE
The source materials used for the production of materials for the manufacture of medicinal products shall be derived from animals fit for human consumption following ante- and post-mortem inspection in accordance with EU or equivalent (third country) conditions, except for materials derived from live animals, which should be found healthy after clinical examination.
3-2-1 Geographical sourcing
3-2-1-1 Bovine materials
The World Organisation for Animal Health (OIE)11 lays down the criteria for the assessment of the status of countries in the chapter of the International Animal Health Code on bovine spongiform encephalopathy. Countries or regions are classified as follows:
As stipulated in Commission Regulation (EC) No 999/2001, as amended12 , the classification of countries or regions thereof according to their BSE risk, based on the rules laid down by OIE, is legally binding in the EU since 1 July 2007. Commission Decision 2007/453/EC13 as amended, provides the classification of countries or regions according to their BSE risk.
Previously, the European Commission Scientific Steering Committee (SSC)14 had established a temporary system for classifying the countries according to their geographical BSE risk (GBR)15 .
For the purposes of this chapter the BSE classification based on the OIE rules should be used. If a country, which was previously classified in accordance to the SSC GBR criteria, has not been classified yet according to the OIE rules, the GBR classification can be used until OIE classification has taken place, provided that there is no evidence of significant change in its BSE risk16 .
Where there is a choice, animals should be sourced from countries with the lowest possible BSE risk (negligible BSE risk countries (Category A)) unless the use of material from countries with a higher BSE risk is justified. Some of the materials identified in Section 6, “Specific Conditions” can be sourced from countries with controlled BSE risk (Category B) and, in some cases, from countries with undetermined BSE risk (Category C), provided that the controls and requirements as specified in the relevant sections below are applied. Apart from these exceptions, animals must not be sourced from countries with undetermined BSE risk (Category C), and justifications for the use of animals from countries with undetermined BSE risk (Category C) must always be provided.
3-2-1-2. Sheep and goats (small ruminants)
Naturally occurring clinical scrapie cases have been reported in a number of countries worldwide. As BSE in sheep and goats could possibly be mistaken for scrapie, as a precautionary measure, sourcing of materials derived from small ruminants shall take into account the prevalence of both BSE and scrapie in the country and the tissues from which the materials are derived.
The principles related to “BSE negligible risk (closed) bovine herds” (see section 3-2-2) could equally be applied in the context of small ruminants in order to develop a framework to define the TSE status of a flock of small ruminants. For sheep, because of the concern over the possibility of BSE in sheep, the use of a genotype(s) showing resistance to BSE/scrapie infection could be considered in establishing TSE free flocks17 . However, the possibility that genotypes resistant to scrapie could be susceptible to BSE (experimental oral exposure) or atypical scrapie (natural cases) should also be taken into account. Goats have not been studied sufficiently with regard to a genotype specific sensitivity.
Material of small ruminant origin should preferably be sourced from countries with a long history of absence of scrapie. Justification shall be required if the material is sourced from some other origin.
3-2-2 BSE negligible risk (closed) bovine herds
The safest sourcing is from countries or regions with a negligible risk (Category A countries). Other countries may have or have had cases of BSE at some point in time and the practical concept of “Negligible risk (closed) bovine herds” has been developed by the SSC and endorsed by the CHMP and CVMP. Criteria for establishing and maintaining a “BSE negligible risk (closed) bovine herd” can be found in the SSC opinion of 22-23 July 199918 .
For the time being it is not possible to quantify the reduction of the geographical BSE risk for cattle from BSE ‘negligible risk (closed) bovine herds’. However, it is expected that this risk reduction is substantial. Therefore, sourcing from such closed bovine herds shall be considered in the risk assessment in conjunction with the OIE classification of the country.
3-3 Animal parts, body fluids and secretions as starting material
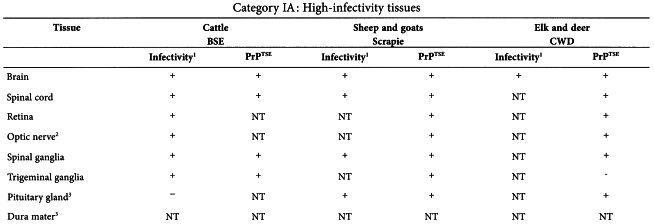
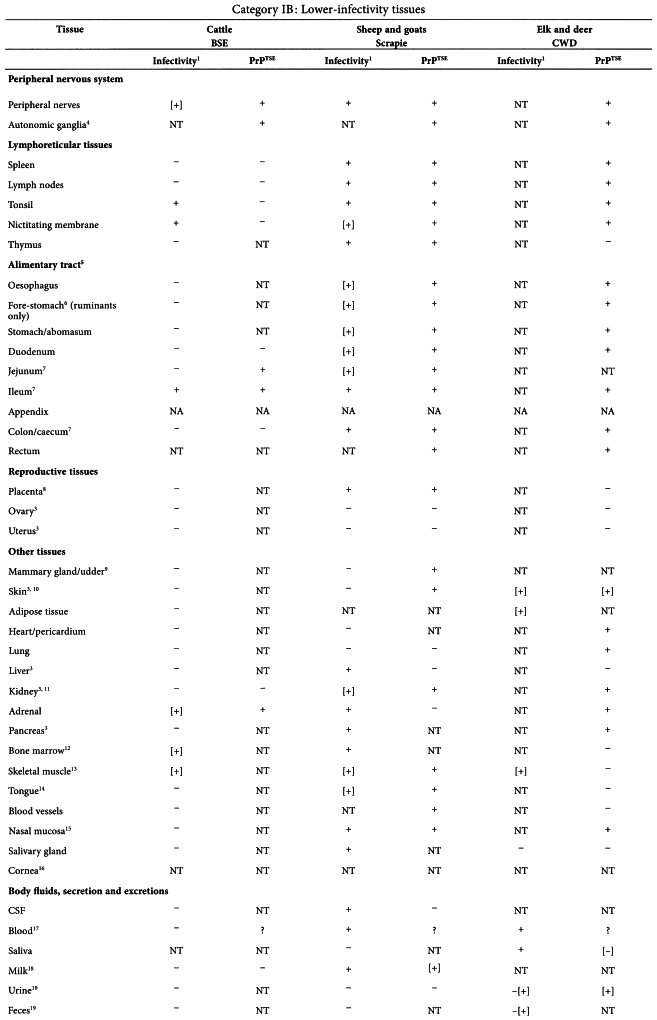
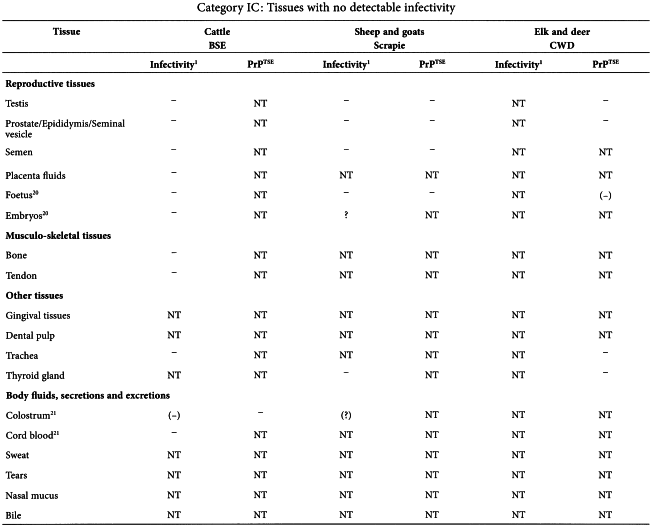
In a TSE infected animal, different organs and secretions have different levels of infectivity. If materials from ‘TSE-relevant animal species’ have to be used, consideration should be given to use materials of the lowest category of risk. The tables given in the Annex of this chapter19 summarise current data about the distribution of infectivity and PrPTSE in cattle with BSE, and in sheep and goats with scrapie20 .
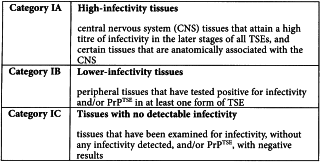
The information in the tables is based exclusively upon observations of naturally occurring disease or primary experimental infection by the oral route (in cattle) but does not include data on models using strains of TSE that have been adapted to experimental animals, because passaged strain phenotypes can differ significantly and unpredictably from those of naturally occurring disease. Because immunohistochemical and/or Western blot detection of misfolded host protein (PrPTSE) have proven to be a surrogate marker of infectivity, PrPTSE testing results have been presented in parallel with bioassay data. Tissues are grouped into three major infectivity categories, irrespective of the stage of disease:
Category IA tissues and substances derived from them shall not be used in the manufacture of medicinal products, unless justified (see Section 5).
Although the category of lower risk tissues (category IB tissues) almost certainly includes some (e.g. blood) with a lower risk than others (e.g. lymphoreticular tissues), the data about infectivity levels in these tissues are too limited to subdivide the category into different levels of risk. It is also evident that the placement of a given tissue in one or another category can be disease and species specific, and subject to revision as new data emerge.
For the risk assessment (see section 4), manufacturers and/or marketing authorisation holders/applicants shall take into account the tissue classification tables in the Annex to this chapter.
The categories in the tables are only indicative and it is important to note the following points.
These factors must be considered in conjunction with the OIE/GBR classification of the source animals, the age of the animals in the case of cattle and the post-mortem testing of the cattle using a validated method.
The underlying principles indicated above would be equally applicable to sheep and goats.
The risk posed by cross-contamination will be dependent on several complementary factors including:
Manufacturers or the marketing authorisation holders/applicants should take into account the risk with respect to cross-contamination.
3-4 Age of animals
As the TSE infectivity accumulates in bovine animals over an incubation period of several years, it is prudent to source from young animals.
Presence of infectious material has essentially been reported in the central nervous system and related tissues, as well as in the lymphoreticular system, depending on the TSE agent (BSE in cattle or scrapie in sheep and goat). The exact time course of infectivity in the respective body parts and tissues, from the date of infection, is not known in both species and, as such, it is difficult to give clear guidance on the age above which the various tissues may be infected and should not be collected. The initial recommendation to collect tissues in the youngest age is still valid. In addition, it is noteworthy that the age criteria depend also on the geographical origin. Age is a more important parameter for materials from countries where the risk is higher (Category B and C countries), than from countries with a negligible BSE risk (Category A countries).
3-5 Manufacturing process
The assessment of the overall TSE risk reduction of a medicinal product shall take into account the control measures instituted with respect to:
Controlled sourcing is a very important criterion in achieving acceptable safety of the product, due to the documented resistance of TSE agents to most inactivation procedures.
A quality assurance system, such as ISO 9000 certification, HACCP22 or GMP, must be put in place for monitoring the production process and for batch delineation (i.e. definition of batch, separation of batches, cleaning between batches). Procedures shall be put in place to ensure traceability as well as self-auditing and to auditing suppliers of raw/starting materials.
Certain production procedures may contribute considerably to the reduction of the risk of TSE contamination, e.g. procedures used in the manufacture of tallow derivatives (see section 6). As such rigorous processing cannot be applied to many products, processes involving physical removal, such as precipitation and filtration to remove prion-rich material, are likely to be more appropriate than chemical treatments. A description of the manufacturing process, including in-process controls applied, shall be presented and the steps that might contribute to reduction or elimination of TSE contamination should be discussed. Whenever different manufacturing sites are involved, the steps performed at each site shall be clearly identified. The measures in place in order to ensure traceability of every production batch to the source material should be described.
Cleaning process
Cleaning of process equipment may be difficult to validate for the elimination of TSE agents. It is reported that after exposure to high titre preparations of TSE agent, detectable infectivity can remain bound to the surface of stainless steel. The removal of all adsorbed protein by the use of 1 M sodium hydroxide or chlorine releasing disinfectants (e.g. 20 000 ppm chlorine for 1 h) have been considered acceptable approaches where equipment that cannot be replaced has been exposed to potentially contaminated material. Milder treatments with limited concentrations of alkali or stabilized bleach, when properly formulated with detergents and used at specified temperatures, have been shown to exhibit similar efficiency for removing prions as did classical NaOH or chlorine treatments. A system based on vaporised hydrogen peroxide also appeared to be efficient for inactivating TSE agents. These new treatments are more compatible with delicate materials and may be suitable for practical use23 .
If risk materials are used in the manufacture of a product, cleaning procedures, including control measures, shall be put in place in order to minimise the risk of cross-contamination between production batches. This is especially important if materials from different risk categories are handled in the same plant with the same equipment. In the case of using category IA materials in the manufacture of a product, dedicated equipment shall be used, unless otherwise justified.
Further research is needed to develop and validate new decontamination procedures to lower the risk of cross-contamination for material and devices which are not compatible with WHO-recommended procedures.
Removal/Inactivation validation
Validation studies of removal/inactivation procedures for TSEs can be difficult to interpret. It is necessary to take into consideration the nature of the spiked material and its relevance to the natural situation, the design of the study (including scaling-down of processes) and the method of detection of the agent (in vitro or in vivo assay). Further research is needed to develop an understanding of the most appropriate “spike preparation” for validation studies. Therefore, validation studies are currently not generally required. However, if claims are made for the safety of the product with respect to TSEs based on the ability of manufacturing processes to remove or inactivate TSE agents, they must be substantiated by appropriate investigational studies 24 .
In addition to appropriate sourcing, manufacturers are encouraged to continue their investigations into removal and inactivation methods to identify steps/processes that would have benefit in assuring the removal or inactivation of TSE agents. In any event, a production process wherever possible shall be designed taking account of available information on methods which are thought to inactivate or remove TSE agents.
For certain types of products (see section 6-3 Bovine blood and blood derivatives), where validated removal/inactivation is not readily applicable, process evaluation might be required. This should be based on the starting material and any published data on TSE risk.
4 RISK ASSESSMENT OF MATERIALS OR SUBSTANCES USED IN THE MANUFACTURE AND PREPARATION OF A MEDICINAL PRODUCT IN THE CONTEXT OF REGULATORY COMPLIANCE
The assessment of the risk associated with TSE needs careful consideration of all of the parameters as outlined in section 3-1 (Scientific Principles for Minimising Risk).
As indicated in the introduction to this chapter, regulatory compliance is based on a favourable outcome from a risk assessment. The risk assessments, conducted by the manufacturers and/or the marketing authorisation holders or applicants for the different materials or substances from “TSE-relevant animal species” used in the manufacture of a medicinal product shall show that all TSE risk factors have been taken into account and, where possible, risk has been minimised by application of the principles described in this chapter. TSE Certificates of suitability issued by the EDQM may be used by the marketing authorisation holders or applicants as the basis of the risk assessments.
An overall risk assessment for the medicinal product, conducted by the marketing authorisation holders or applicants, shall take into account the risk assessments for all the different materials from “TSE-relevant animal species” and, where appropriate, TSE reduction or inactivation by the manufacturing steps of the active substance and/or finished product.
The final determination of regulatory compliance rests with the competent authority.
It is incumbent upon the manufacturers and/or the marketing authorisation holders or applicants for both human and veterinary medicinal products to select and justify the control measures for a given “TSE-relevant animal species” derivative, taking into account the latest scientific and technical progress.
5 Benefit/risk evaluation
In addition to the parameters as mentioned in sections 3 (that may be covered by a TSE Certificate of Suitability issued by the EDQM) and 4, the acceptability of a particular medicinal product containing materials derived from a “TSE-relevant animal species”, or which as a result of manufacture could contain these materials, shall take into account the following factors:
High-infectivity tissues (category IA tissues) and substances derived thereof shall not be used in manufacture of medicinal products, their starting materials and intermediate products (including active substances, excipients and reagents), unless justified. A justification why no other materials can be used shall be provided. In these exceptional and justified circumstances, the use of high-infectivity tissues could be envisaged for the manufacture of active substances, when, after performing the risk assessment as described in Section 4 of this chapter, and taking into account the intended clinical use, a positive benefit/risk assessment can be presented by the marketing authorisation applicant. Substances from category IA materials, if their use is justified, must be produced from animals of countries with negligible BSE risk (Category A).
6 Specific considerations
The following materials prepared from “TSE-relevant animal species” are considered in compliance with this chapter provided that they meet at least the conditions specified below. The relevant information or a certificate of suitability granted by the EDQM shall be provided by the marketing authorisation applicant/holder.
6-1 COLLAGEN
Collagen is a fibrous protein component of mammalian connective tissue.
For collagen, documentation to demonstrate compliance with this chapter needs to be provided taking into account the provisions listed in sections 3 to 5. In addition, consideration should be given to the following.
The collagen manufacturing process can have some steps in common with the manufacture of gelatin such as alkaline and sodium sulphate treatment, calcium hydroxide and sodium hydroxide treatments or enzyme treatment. However, even these common steps can differ in duration and pH condition which can result in significant differences in their inactivation capacity. Manufacturers should at least conduct a process evaluation based on the similarities of the collagen processing steps, as compared to known inactivation steps in the manufacture of gelatin, in order to support the safety of the product. In addition to processing, differences also exist in the final use of the material and, consequently, in their risk assessment, while gelatin is widely used for oral administration, many collagen applications are in the form of surgical implants. This aspect should also be considered in the final risk assessment.
6-2 GELATIN
Gelatin is a natural, soluble protein, gelling or non-gelling, obtained by the partial hydrolysis of collagen produced from bones, hides and skins of animals.
For gelatin, documentation to demonstrate compliance with this chapter needs to be provided taking into account the provisions listed in sections 3 to 5. In addition, consideration should be given to the following25 .
The requirements for source material selection and manufacture are appropriate for oral or parenteral gelatin for use in human and veterinary medicinal products.
The requirements for source material selection and manufacture are appropriate for oral or parenteral gelatin for use in human and veterinary medicinal products.
The source material used
Gelatin used in medicinal products can be manufactured from bones or hides.
Hides as the starting material On the basis of current knowledge, hides used for gelatin production represent a safer source material as compared to bones. However, it is highly recommended that measures should be put in place to avoid cross-contamination with potentially infected materials during procurement.
Bones as the starting material Where bones are used to manufacture gelatin, the quality of the starting materials needs to be controlled as an additional parameter to ensure the safety of the final product. Therefore, the following should be applied.
Manufacturing methods
Hides No specific measures with regard to the processing conditions are required for gelatin produced from hides provided that control measures are put in place to avoid cross-contamination both during the procurement of the hides and during the manufacturing process.
Bones Where bones are used as the starting material, the mode of manufacture will be the second parameter that will ensure the safety of gelatin.
The finishing steps are similar for the alkaline, acid and heat/pressure process and include extraction of the gelatine, washing, filtration and concentration.
6-3 BOVINE blood and BLOOD DERIVATIVES
Foetal bovine serum is commonly used in cell cultures. Foetal bovine serum should be obtained from foetuses harvested in abattoirs from healthy dams fit for human consumption and the womb should be completely removed and the foetal blood harvested in dedicated space or area by cardiac puncture into a closed collection system using aseptic technique.
Newborn calf serum is obtained from calves under 20 days old and calf serum from animals under the age of 12 months. In the case of donor bovine serum, given that it may be derived from animals less than 36 months old, the TSE negative status of the donor herd shall be well defined and documented. In all cases, serum shall be collected according to specified protocols by personnel trained in these procedures to avoid cross-contamination with higher risk tissues.
For bovine blood and blood derivatives, documentation to demonstrate compliance with this chapter needs to be provided taking into account the provisions listed in sections 3 to 5. In addition, consideration should be given to the following.
Traceability
Traceability to the slaughterhouse must be assured for each batch of serum or plasma. Slaughterhouses must have available lists of farms from which the animals are originated. If serum is produced from living animals, records must be available for each serum batch which assures the traceability to the farms.
Geographical origin
Whilst tissue infectivity of BSE in cattle is more restricted than scrapie, as a precautionary measure bovine blood should be sourced from Category A countries. Bovine blood from Category B countries is also acceptable provided that there is no risk for cross-contamination of blood with brain material from the slaughter of animals over 21 months26 of age.
Stunning methods
If it is sampled from slaughtered animals, the method of slaughter is of importance to assure the safety of the material. It has been demonstrated that stunning by captive bolt stunner with or without pithing as well as by pneumatic stunner, especially if it injects air, can destroy the brain and disseminate brain material into the blood stream. Non-penetrative stunning is no more considered as an alternative to penetrative stunning because contamination of blood with brain material has been demonstrated27 . Negligible risk can be expected from electro-narcosis28 , but this even does not provide strict safety because, when unsuccessful, animals may have to be additionally stunned. The stunning methods must therefore be described for the bovine blood collection process.
Whenever a risk of cross-contamination of blood with brain cannot be avoided at routine slaughtering in countries with a controlled BSE risk (Category B), safety measures such as restriction of the age of the cattle and/or reduction of infectious agents during manufacture have to be applied.
Age
For countries with a controlled BSE risk (Category B), a precautionary age limit of 21 months shall apply for bovine blood or blood derivatives where no significant reduction of TSE agents can be assumed from manufacture. An age limit of 30 months is considered sufficient for blood derivatives where significant reduction of TSE agents can be demonstrated as described below.
Reduction of TSE agents during manufacture
For blood derivatives, the capacity of the manufacturing process to reduce/eliminate TSE agents should be estimated from investigational studies. The estimation may be based on published data or in house data whenever it can be shown that such data is relevant to the specific manufacturing process. If it cannot be concluded that the reduction capacity is comparable, it is recommended that manufacturers undertake product-specific investigational studies. Investigations using biochemical assay may be sufficient if there is scientific evidence that this assay correlates with infectivity data. General guidance for investigational studies on reduction of TSE agents has been outlined29 . Brain-derived spike preparations are appropriate for studies investigating the risk from brain-contaminated blood.
6-4 TALLOW DERIVATIVES
Tallow is fat obtained from tissues including subcutaneous, abdominal and inter-muscular areas and bones. Tallow used as the starting material for the manufacture of tallow derivatives shall be ‘Category 3 material or equivalent’, as defined in Regulation (EC) No 1774/2002 of the European Parliament and of the Council of 3 October 2002 laying down health rules concerning animal by-products not intended for human consumption.
Tallow derivatives, such as glycerol and fatty acids, manufactured from tallow by rigorous processes are thought unlikely to be infectious and they have been the subject of specific consideration by CHMP and CVMP. For this reason, such materials manufactured under the conditions at least as rigorous as those given below shall be considered in compliance for this chapter, irrespective of the geographical origin and the nature of the tissues from which tallow derivatives are derived. Examples of rigorous processes are:
Tallow derivatives manufactured according to these conditions are unlikely to present any TSE risk and shall therefore be considered compliant with this chapter.
Tallow derivatives produced using other conditions must demonstrate compliance with this chapter.
6-5 ANIMAL CHARCOAL
Animal charcoal is prepared by carbonisation of animal tissues, such as bones, using temperatures higher than 800 °C. Unless otherwise justified, the starting material for the manufacture of animal charcoal shall be Category 3 material or equivalent, as defined in Regulation (EC) No 1774/2002 of the European Parliament and of the Council of 3 October 2002 laying down health rules concerning animal by-products not intended for human consumption. Irrespective of the geographical origin and the nature of the tissue, for the purpose of regulatory compliance, animal charcoal shall be considered in compliance with this chapter.
Charcoal manufactured according to these conditions is unlikely to present any TSE risk and shall therefore be considered compliant with this chapter. Charcoal produced using other conditions must demonstrate compliance with this chapter.
6-6 MILK AND MILK DERIVATIVES
In the light of the current scientific knowledge and irrespective of the geographical origin, bovine milk is unlikely to present any risk of TSE contamination30 .
Certain materials, including lactose, are extracted from whey, the spent liquid from cheese production following coagulation. Coagulation can involve the use of calf rennet, an extract from abomasum, or rennet derived from other ruminants. The CHMP/CVMP have performed a risk assessment for lactose and other whey derivatives produced using calf rennet and concluded that the TSE risk is negligible if the calf rennet is produced in accordance with the process described in the risk assessment report31 . The conclusion was endorsed by the SSC32 which has also per formed an assessment of the TSE risk of rennet in general33 .
Bovine milk derivatives manufactured according to the conditions described below are unlikely to present any TSE risk and shall therefore be considered compliant with this chapter.
Milk derivatives produced using other processes or rennet derived from other ruminant species must demonstrate compliance with this chapter.
6-7 WOOL DERIVATIVES
Derivatives of wool and hair of ruminants, such as lanolin and wool alcohols derived from hair shall be considered in compliance with this chapter, provided the wool and hair are sourced from live animals.
Wool derivatives produced from wool which is sourced from slaughtered animals declared “fit for human consumption” and the manufacturing process in relation to pH, temperature and duration of treatment meets at least one of the stipulated processing conditions listed below are unlikely to present any TSE risk and shall therefore be considered compliant with this chapter.
Wool derivatives produced using other conditions must demonstrate compliance with this chapter.
6-8 AMINO ACIDS
Amino acids can be obtained by hydrolysis of materials from various sources.
Unless otherwise justified, the starting material for the manufacture of amino acids shall be ‘Category 3 material or equivalent’, as defined in Regulation (EC) No 1774/2002 of the European Parliament and of the Council of 3 October 2002 laying down health rules concerning animal by-products not intended for human consumption.
Amino acids prepared using the following processing conditions are unlikely to present any TSE risk and shall be considered compliant with this chapter:
Amino acids prepared using other conditions must demonstrate compliance with this chapter.
6-9 PEPTONES
Peptones are partial hydrolysates of protein, achieved by enzymic or acid digestion. They are used in microbiological culture media to support the nutritional requirements of micro-organisms, which might be used as seed stocks or in industrial scale fermentations for the production of human and veterinary medicinal products, including vaccines. There is considerable interest in the use of vegetable protein as an alternative to animal sourced protein. However:
Annex: major categories of infectivity
The tables below are taken from the WHO Guidelines on Tissue Infectivity Distribution in Transmissible Spongiform Encephalopathies (2010).
Data entries are shown as follows:
| + | = | Presence of infectivity or PrPTSE |
| - | = | Absence of detectable infectivity or PrPTSE |
| NT | = | Not tested |
| NA | = | Not applicable |
| ? | = | Uncertain interpretation |
| () | = | Limited or preliminary data |
| [] | = | Infectivity or PrPTSE data based exclusively on bioassays in transgenic (Tg) mice over-expressing the PrP-encoding gene or PRPTSE amplification methods |
Reports of the GBR assessment of the countries are available on the SSC website (http://ec.europa.eu/food/fs/sc/ssc/outcome_en.html)