Betahistine Dihydrochloride Tablets
Action and use
Histamine H1 receptor antagonist; antihistamine.
Definition
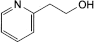
Betahistine Dihydrochloride Tablets contain Betahistine Dihydrochloride.
Content of betahistine dihydrochloride, C8H12N2,2HCl
95.0 to 105.0% of the stated amount.
Identification
Tests
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
Dissolve 0.4 g of hexylamine in 600 mL of a solution containing 0.46% w/v of sodium dihydrogen orthophosphate monohydrate and 0.27% w/v of sodium dodecyl sulfate, add 400 mL of acetonitrile, mix and adjust the pH to 3.5 using orthophosphoric acid.
The test is not valid unless, in the chromatogram obtained with solution (5), the resolution factor between the two principal peaks is at least 3.0.
In the chromatogram obtained with solution (1):
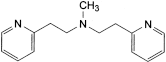
the area of any peak corresponding to N-methyl-bis[β-(2-pyridyl)ethyl]amine is not greater than the area of the principal peak in the chromatogram obtained with solution (3) (2%);
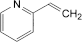
the area of any peak corresponding to 2-vinylpyridine is not greater than twice the area of the principal peak in the chromatogram obtained with solution (4) (0.2%);
the area of any other secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.2%);
the sum of the areas of the peaks corresponding to N-methyl-bis[β-(2-pyridyl)ethyl]amine, 2-vinylpyridine and any other secondary peaks is not greater than 10 times the area of the principal peak in the chromatogram obtained with solution (2) (2%).
Disregard any peak with an area less than 0.05% of that of the principal peak in the chromatogram obtained with solution (1) (0.05%).
Assay
Weigh and powder 20 tablets. Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
The chromatographic conditions described under Related substances may be used.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the two principal peaks is at least 3.0.
Calculate the content of C8H12N2,2HCl in the tablets using the declared content of C8H12N2,2HCl in betahistine dihydrochloride BPCRS.
Storage
Betahistine Dihydrochloride Tablets should be protected from light and moisture.