Levonorgestrel and Ethinylestradiol Tablets
Action and use
Progestogen.
Definition
Levonorgestrel and Ethinylestradiol Tablets contain Levonorgestrel and Ethinylestradiol.
Content of levonorgestrel, C21H28O2
90.0 to 110.0% of the stated amount.
Content of ethinylestradiol, C20H24O2
90.0 to 110.0% of the stated amount.
Identification
Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions.
1 volume of methanol and 99 volumes of chloroform.
By the first method of visualisation:
one of the principal spots in the chromatogram obtained with solution (1) corresponds to that in the chromatogram obtained with solution (2) and the other principal spot corresponds to that in the chromatogram obtained with solution (3).
By the second method of visualisation:
the spots corresponding to levonorgestrel and ethinylestradiol appear as blue spots.
Uniformity of content
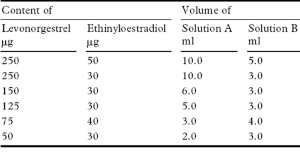
Prepare the following stock solutions using a mixture of 40 volumes of water and 60 volumes of acetonitrile.
Tablets containing less than 2 mg and/or less than 2% w/w of Levonorgestrel or less than 2 mg and/or less than 2% w/w of Ethinylestradiol comply with the requirements stated under Tablets using the following method of analysis. Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
49 volumes of acetonitrile and 51 volumes of water.
Calculate the content of C21H28O2 and of C20H24O2 in each tablet using the declared content of C21H28O2 in levonorgestrel BPCRS and the declared content of C20H24O2 in ethinylestradiol BPCRS.
Assay
For both levonorgestrel and ethinylestradiol use the average of the individual results determined in the test for Uniformity of content.