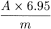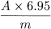Cascara Tablets
Definition
Cascara Tablets contain Standardised Cascara Dry Extract. They are coated.
Content of total hydroxyanthracene derivatives
17.0 to 23.0 mg, of which not less than 60% consists of cascarosides, both expressed as cascaroside A.
Identification
Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions.
13 volumes of water, 17 volumes of methanol and 100 volumes of ethyl acetate.
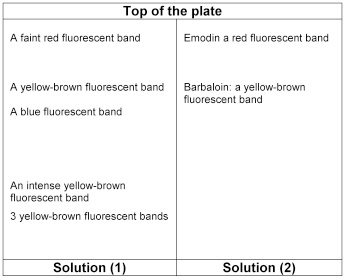
The chromatogram obtained with solution (1) show yellowish-brown fluorescent bands with Rf values of between 0.2 and 0.25, an intense yellowish-brown fluorescent band with an Rf value of about 0.3, a blue fluorescent band with an Rf value of about 0.6, a yellowish-brown fluorescent band with an Rf value of about 0.7 corresponding in colour and position to the band obtained with barbaloin in solution (2) and a faint reddish fluorescent band with an Rf value of about 0.9 corresponding in position to emodin in solution (2).
Tests
Disintegration
Comply with the requirements stated under Tablets but for sugar-coated tablets the maximum time is 120 minutes.
Assay
Carry out the assay within 24 hours, protected from bright light.
Add 80 mL of 70% v/v ethanol to a quantity of the powdered tablets containing 75 mg of total hydroxyanthracene derivatives. Shake and allow to stand in the dark for at least 8 hours. Dilute to 100.0 mL with 70% v/v of ethanol. Shake and filter, discarding the first 20 mL of filtrate. Transfer 10.0 mL of the filtrate to a separating funnel, add 0.1 mL of 1m hydrochloric acid and shake with 2-quantities, each of 20 mL, of a mixture of 1 volume of ether and 3 volumes of hexane. Wash the combined organic extracts with 5 mL of water. Discard the organic layer and return the rinsings to the hydroalcoholic layer. Shake with 4-quantities, each of 30 mL, of ethyl acetate freshly saturated with water prepared by shaking 150 mL of ethyl acetate with 15 mL of water for 3 minutes and allowing to stand until the layers have separated and the organic layer is clear. Combine the ethyl acetate extracts and use the aqueous layer for the assay of cascarosides and the organic layer for the assay of hydroxyanthracene glycosides other than cascarosides.
Hydroxyanthracene glycosides other than cascarosides
Transfer the organic layer to a round-bottomed flask and remove the solvent by distillation, evaporating almost to dryness. Dissolve the residue in 0.5 mL of methanol, add 10 mL of water at 40° and transfer to a 50 mL volumetric flask, rinsing the round-bottomed flask with water at 40° and adding the rinsings to the hydromethanolic solution. Allow to cool and dilute to 50.0 mL with water. Transfer 20.0 mL of the solution to a 100 mL round-bottomed flask with a ground-glass neck containing 2 g of iron (iii) chloride hexahydrate and 12 mL of 7m hydrochloric acid. Attach a reflux condenser and place the flask in a water-bath so that the level of the water is above that of the liquid in the flask and heat for 4 hours. Allow to cool, transfer the solution to a separating funnel and rinse the flask successively with 4 mL of 1m sodium hydroxide and 4 mL of water, adding the rinsings to the separating funnel. Shake the contents of the separating funnel with 3-quantities, each of 30 mL, of a mixture of 1 volume of ether and 3 volumes of hexane. Wash the combined organic layers with 2-quantities, each of 10 mL, of water and discard the rinsings. Dilute the organic layer to 100.0 mL with a mixture of 1 volume of ether and 3 volumes of hexane. Take 20.0 mL of the solution, evaporate carefully to dryness on a water-bath and dissolve the residue in 10.0 mL of a 0.5% w/v solution of magnesium acetate in methanol. Measure the absorbance of the resulting solution at 440 nm and at 515 nm, Appendix II B, using methanol in the reference cell. The assay is not valid if the ratio of the absorbance at 515 nm to that at 440 nm is less than 2.4.
Calculate the percentage content of hydroxyanthracene glycosides other than cascarosides, expressed as cascaroside A, using the following expression:
i.e. taking the specific absorbance to be 180.
A | = | absorbance at 515 nm; |
m | = | weight of the substance being examined, in grams. |
Cascarosides
To the aqueous solution reserved from the preliminary extraction add sufficient water to produce 50.0 mL. Carry out the Assay for hydroxyanthracene gycosides other than cascarosides, beginning at the words, ‘Transfer 20 mL …’. Measure the absorbance of the resulting solution at 440 nm and at 515 nm, Appendix II B, using methanol in the reference cell. The assay is not valid if the ratio of the absorbance at 515 nm to that at 440 nm is less than 2.7.
Calculate the percentage content of cascarosides, expressed as cascaroside A, using the following expression:
i.e. taking the specific absorbance to be 180.
A | = | absorbance at 515 nm; |
m | = | weight of the substance being examined, in grams. |
Labelling
The label states the nominal content of hydroxyanthracene glycosides, expressed as cascarosides A.