SC I L. Microbiological Assay of Antibiotics
This section provides guidance on interpretation of statements in the Pharmacopoeia concerning content limits for those antibiotics and their preparations for which the monograph specifies a microbiological assay.
1. The statements in the Pharmacopoeia concerning the potency of antibiotics are framed to provide a control analyst with the means whereby he can ascertain whether or not the material or preparation in question is satisfactory, that is, they provide criteria appropriate to a ‘check assay’.
2. In order to be confident that, when assayed by a control analyst, a material or preparation would meet the pharmacopoeial criteria a manufacturer would himself need to work to criteria appropriate to a ‘release assay’.
3. The need for different criteria for check and release assay purposes arises from the inherent variability of biological systems which precludes assigning an exact value to the true potency. It is possible only to give a range of values within which the true potency can be expected to lie with a defined degree of confidence (95%; P = 0.95 for pharmacopoeial purposes). In such circumstances the fiducial limits of error computed for the assay in question define the lower and upper limits within which the true potency of the sample lies, with a probability of 95%.
4. The required minimum precision for an acceptable assay of any particular antibiotic or preparation is defined in the appropriate monograph in the paragraph entitled Assay. This degree of precision is the minimum acceptable for determining that the final product complies with the official requirements. It may be inadequate for a decision about the potency that should be stated on the label or used as the basis for calculating the quantity of an antibiotic to be incorporated in a preparation. In such circumstances, assays of greater precision may be desirable with, for instance, fiducial limits of error of the order of 98 to 102%. With this degree of precision, the lower fiducial limit lies close to the estimated potency. By using this limit, instead of the estimated potency, to assign a potency to the antibiotic either for labelling or for calculating the quantity to be included in a preparation, there is less likelihood of the final preparation subsequently failing to comply with the official requirements for potency. Greater precision can be achieved by statistically combining the results of two or more independent assays.
Bulk antibiotic
5. In a monograph for a bulk antibiotic a minimum potency in IU is given under the heading Definition and this is to be interpreted in accordance with the second paragraph of the General Notice on Biological Assays and Tests. This states that ‘The material is not of pharmacopoeial quality if the upper fiducial limit of error is less than the stated potency. For such antibiotics the required precision of the assay is stated in terms of the fiducial limits about the estimated potency.’
6. Taking Amphotericin, the monograph for which has a defined minimum potency of not less than 750 IU per mg, as an example of a bulk antibiotic: a control analyst would judge the material unsatisfactory with respect to potency only if the upper fiducial limit of error [UFLE] obtained in his assay was less than 750 IU per mg, calculated with reference to the dried substance. A manufacturer, on the other hand, would consider suitable for release only those batches for which the lower fiducial limit of error [LFLE] obtained in his assay was greater than 750 IU per mg, calculated with reference to the dried substance.
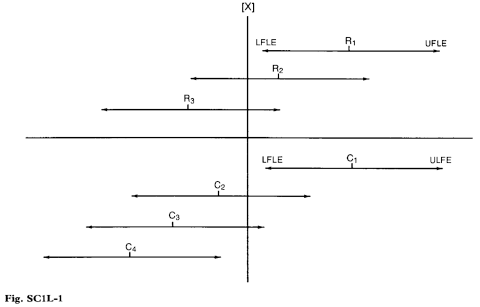
Bulk antibiotic (Fig. SC1L–1)
Probability = 0.95,
Precision = 95–105%
Let X be the monograph value for the minimum potency of the bulk antibiotic [750 IU per mg, for example, for Amphotericin].
Let R1, R2, R3 be the estimated potency found by the manufacturer in the release assay.
To ensure (with 95% confidence) that the true potency of his bulk substance is not less than the desired minimum [ie that R not <X], the manufacturer releases only a batch such as R1 for which the lower fiducial limit of error of the estimated potency is ≥X; he rejects batches such as R2 and R3 for which the lower fiducial limit of error is <X.
Let C1, C2, C3, C4 be the estimated potency found by the control analyst in the check assay.
Only when the upper fiducial limit of error of the estimated potency is <X can the control analyst conclude (with 95% confidence) that the true potency of the sample being examined is less than the desired minimum value. He would fail a batch with estimated potency C4.
Formulated preparation
7. In a monograph for a formulated preparation the lower and upper limits of content are given under the heading Assay and are given as the values (stated in terms of percentage of labelled claim) within which the fiducial limits of error of the estimated potency obtained in the Assay must lie.
8. For a formulated preparation the results of the Assay and the content, or strength, stated on the label are given in terms of IU unless specific instruction to calculate a ‘weight equivalent’ is given as, for example, in the monograph for Streptomycin Injection. Such a ‘weight equivalent’ cannot be given for certain antibiotics, in particular those like Neomycin Sulfate which consist of a variable mixture of components.
9. Taking Neomycin Eye Ointment, the monograph for which requires the upper fiducial limit of error to be not less than 90% and the lower fiducial limit of error to be not more than 115% of the stated amount, as an example of a formulated preparation: a control analyst would judge a product unsatisfactory with respect to content only if the upper fiducial limit of error obtained in the check assay was less than 90% or if the lower fiducial limit of error was greater than 115% of the amount expected in accordance with the strength stated on the label. A manufacturer, on the other hand, would consider suitable for release only those batches of the product for which the lower fiducial limit of error obtained in his assay was greater than 90% and for which the upper fiducial limit of error was less than 115% of the amount expected in accordance with the strength stated on the label.
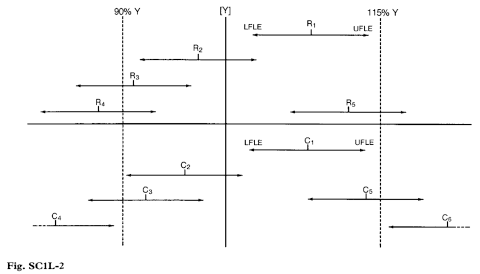
Formulated preparation (Fig. SC1L-2)
Probability = 0.95,
Precision = 95 – 105%
Let Y be the stated content/strength [labelled claim] of the preparation [3500 IU per g of ointment, for example, for Neomycin Eye Ointment].
Let R1, R2, R3, R4, R5 be the estimated content/strength found by the manufacturer in the release assay.
To ensure (with 95% confidence) that the true content/strength is not less than the specified minimum [ie that R not <90% Y], the manufacturer releases only batches such as R1 and R2 for which the lower fiducial limit of error of the estimated potency is ≥90% Y; he rejects batches such as R3 and R4 for which the lower fiducial limit of error is <90% Y. To ensure (with 95% confidence) that the true content/strength is not greater than the specified maximum [ie that R not >115% Y], the manufacturer also rejects batches such as R5 for which the upper fiducial limit of error is >115% Y. In order to avoid possible problems of compliance with monograph assay limits at the end of shelf-life for those antibiotics where stability is an issue, manufacturers are advised to exercise caution in releasing batches such as R2 for which the lower fiducial limit of error is less than the value stated on the label. The advice given in paragraph 4 may be of assistance in these circumstances.
Let C1, C2, C3, C4, C5, C6 be the estimated content/strength found by the control analyst in the check assay.
Only when the lower fiducial limit of error of the estimated content/strength is >115% Y or when the upper fiducial limit of error of the estimated content/strength is <90% Y can the control analyst conclude (with 95% confidence) that the true content/strength is outside the specified range; he would fail batches with estimated content/strength C4 and C6.
It is important to recognise that, for the reasons explained in paragraph 3, a batch giving the estimate R4 in a release assay may give the estimate C4 in a check assay without any change in the true potency.
Definitions
Assay A single test carried out for the purpose of estimating the potency of a material/preparation or the pooled results of two or more such tests.
True potencyThe actual potency of the material/preparation at the time of assay. In practice this value can never be exactly evaluated.
Stated or labelled potency This is often a nominal value assigned to a formulated preparation from knowledge of the potency of the bulk material. In the case of bulk materials, it may be calculated from assay data.
Estimated potency The potency calculated from assay data. It is misleading to refer to a ‘best estimate’ [see under fiducial limits below].
Fiducial limits [confidence interval] These are limits to the true potency of the material/preparation and are calculated on the assumption that there is no bias in the assay system. They may be calculated for any desired level of probability; for pharmacopoeial purposes a level of 0.95 is applied. The concept is that, if the assay could be repeated many times, the true potency would lie within the fiducial limits in 95% of assays. Thus, in any particular assay, the true potency could be anywhere within the fiducial range (and occasionally outside it) and the estimated potency is no better measure of the true potency than any other value in the range.
Release and check assays A release assay is an assay carried out by those responsible for assigning a potency to a material or declaring a content or strength for a preparation [manufacturers]. A check assay is an assay carried out by those responsible for checking the potency of a material or the content or strength of a preparation [control authorities, independent analysts and purchasers of bulk materials]. The use of these two terms does not imply a fundamental difference in the design or execution of an assay but rather in the interpretation of the results obtained. A check assay only enables a control analyst to say unequivocally when a material/preparation is unsatisfactory [see illustration above].