These notes provide a guide to the semi-systematic chemical nomenclature of certain groups of natural and semi-synthetic products. The following literature should be consulted for further information.
1. The definitive rules of the International Union of Pure and Applied Chemistry (
IUPAC) for organic substances are contained in
Nomenclature of Organic Chemistry, Section A to F and H, Pergamon Press, Oxford, 1979.
2. The
IUPAC rules for inorganic substances are given in
Nomenclature of Inorganic Chemistry, Blackwells, Oxford, 1990.
3. Biochemical Nomenclature and Related Documents, The Biochemical Society for the International Union of Biochemistry (IUB), 2nd edn. London, 1992. The following topics are included.
Stereochemistry (Nomenclature of Organic Chemistry, Section E)
Natural products and related compounds (Nomenclature of Organic Chemistry, Section F)
Abbreviations and Symbols
Amino acids, peptides, peptide hormones and immunoglobulins
Steroids
Carotenoids and tocopherols
Carbohydrates and cyclitols
Vitamins
A. Aminoglycoside Antibiotics
A.1 The aminoglycoside antibiotics are conveniently named by reference to 2-d-deoxystreptamine, 1, in which name the configuration and numbering shown are implicit.

A.2 The aminoglycoside antibiotics commonly carry glycosyl radicals on the oxygen atoms attached to C-4 and C-6. The configuration and numbering shown in 1 should be strictly observed if confusion is to be avoided.
A.3 When one glycosyl radical is linked to another the names are separated by two locants which indicate the respective positions involved in this glycosidic union; these locants are enclosed in parentheses and separated by an arrow (pointing from the locant corresponding to the glycosyl carbon atom to the locant corresponding to the hydroxylic carbon atom involved).
B. Cephalosporin Antibiotics
B.1 The cephalosporin antibiotics are conveniently named by reference to either cephalosporanic acid (2, R = CH 2OAc, X = H) or cephem-4-carboxylic acid (2, R = X = H).
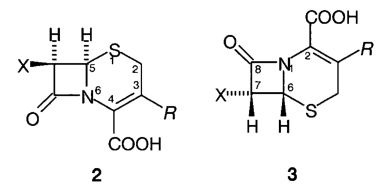
B.2 When names are based on cephalosporanic acid or cephem-carboxylic acid the traditional numbering shown in 2 is used.
B.3 Cephalosporanic acid is systematically named as (6R)-3-acetoxymethyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. Compounds that are named systematically use the numbering and orientation shown in 3.
B.4 The cephalosporin antibiotics bear an acylamino group (X) at position 7 (under both numbering systems).
C. Ergot Alkaloids
C.1 Members of the ergoline group of substances are conveniently named by reference to either ergoline itself, 4, or to d-lysergamide, 5. When names are so based the traditional numbering shown in 4 is used. In 9,10-dihydro compounds the configuration at position-10 needs to be specified.
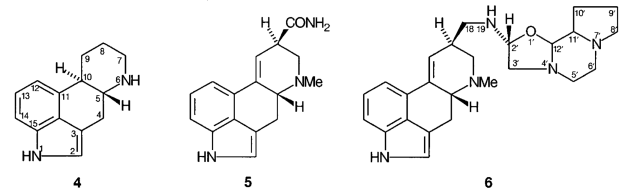
C.2 Members of the ergotamine group of substances are conveniently named by reference to ergotaman, 6. Ergotamine itself is (5′R)-5′-benzyl-12′-hydroxy-2′-methyl-18-oxoergotaman-3′,6′-dione.
D. Morphines
D.1 Members of the morphine and codeine group of substances have traditionally been named with reference to morphine itself, 7, using the numbering shown. However, names may be based more conveniently on either morphinan, 8, or ent-morphinan, 9.
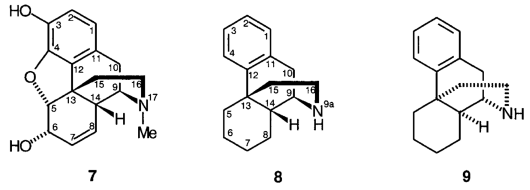
D.2 Morphine, 7, is (5R, 6S)-4,5-epoxy-9a-methyl-7,8-didehydromorphinan-3,6-diol.
D.3 In certain morphine derivatives, an etheno or ethano bridge is present joining positions 6 and 14 and a hydroxyalkyl side chain is present at position 7.
E. Penicillin Antibiotics
E.1 The penicillin antibiotics are conveniently named by reference to penicillanic acid (10, X = H) when the classical numbering shown is used.
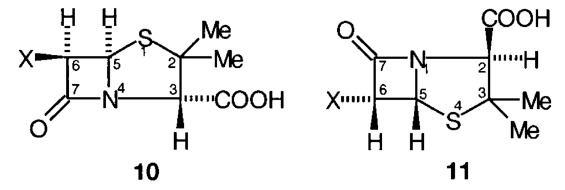
E.2 Penicillanic acid is systematically named as (2S, 5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid. Compounds that are named systematically use the orientation and numbering shown in 11.
E.3 The penicillin antibiotics are usually 6-acylamino penicillanic acid derivatives in which the configuration at position 6 is R.
F. Polypeptides
F.1 The following 3-letter and 1-letter symbols for amino acids, authorised by the
IUPAC-IUB Joint Commission on Biochemical Nomenclature, are used for representing the sequences of polypeptides:
F.2 The following symbols recommended by the Joint Commission are also used:
2-Aminohexanoic acid | Ahx |
Sarcosine | Sar |
Pyroglutamic acid | <Glu |
tert-Butoxycarbonyl | Boc |
F.3 When interpreting sequences of amino-acid residues, the hyphen should be considered as part of the symbol. Its use to separate the individual residues or radicals may be illustrated by the following example:
Gly | = | NH2CH2COOH |
Gly- | = | NH2CH2CO– |
–Gly | = | –NHCH2COOH |
–Gly- | = | –NHCH2CO– |
and thus, | | |
Gly-Gly-Gly | = | NH2CH2CONHCH2CONHCH2COOH |
The residues are conventionally written with the amino group to the left and the carboxyl group to the right. This is implicit in the symbolism.
F.4 Where peptide sequences are shown using 3-letter symbols all amino acids except glycine have the ‘l-’ configuration unless otherwise indicated.
G. Prostaglandins
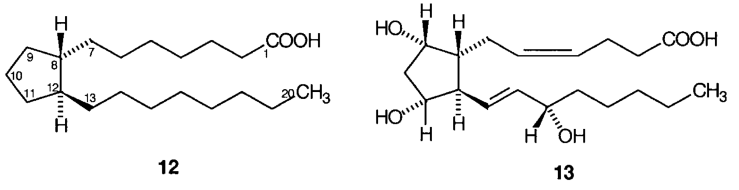
G.1 Members of the prostaglandin group of substances are conveniently named by reference to prostanoic acid, 12, using the numbering shown. Prostanoic acid may be systematically named as 7-[(1S, 2S)-2-octylcyclopentyl]heptanoic acid. Dinoprost, 13, may therefore be named as (5Z,12E)-(9S, 11R, 15S)-9, 11,15-trihydroxyprosta-5,13-dienoic acid.
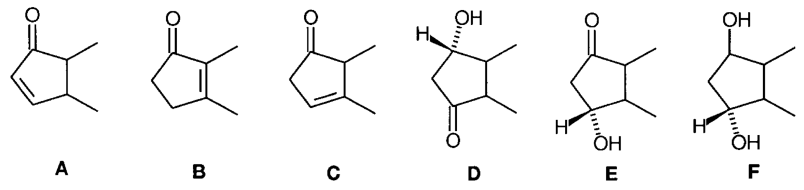
G.2 A convenient trivial nomenclature exists by which prostaglandins (PG) are classified into groups A to F according to the substitution pattern in the cyclopentane ring (shown below). The subscript numerals 1, 2 and 3 refer to the number of double bonds found in the side-chains and subscript a refers to the configuration of the C-9 hydroxy group. Thus, 13 is referred to as PGF2a.
H. Steroids
H.1 Steroids are drawn and numbered, and the rings lettered, as shown in 14.
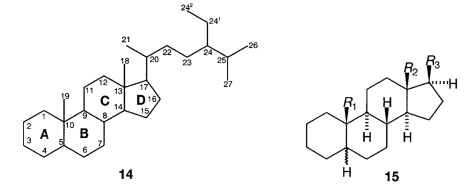
H.2 Steroids are named with reference to certain basic carbocycles some of which are defined in Table I. When so drawn, dotted bonds are regarded as lying below the plane of the paper and are designated a, thickened bonds are regarded as lying above the plane of the paper and are designated b and bonds of unknown configuration are shown by a wavy line and are designated x.

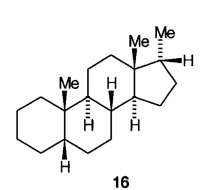
H.3 When the hydrogen atom at C-5 is present, its configuration is always specified, eg 5a-pregnane, 5b-androstane. The configuration at centres, 8,9,10,13,14 and 17 is assumed to be as shown in 15 unless otherwise specified.
H.4 When inversion of the normal configuration occurs, the positions concerned are specified; and thus 16 is named 5b,17a-pregnane.
H.5 When inversion occurs at all of the defined asymmetric centres, the original name is preceded by the italicised prefix ent-. Racemates are indicated by use of the italicised prefix rac-.
H.6 Further fundamental carbocycles are defined thus:
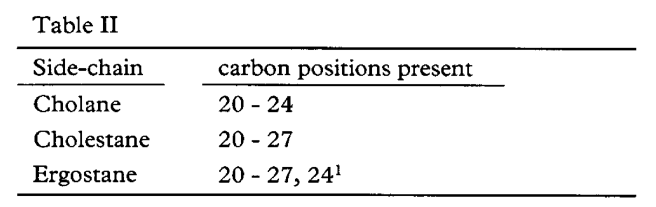
In addition to retaining the configuration shown in 15, C-20 has an R-configuration in each carbocycle and C-24 in ergostane has an S-configuration. However, additional substituents at positions C-17, C-20, C-21 may alter the R and S propriety descriptions without any change at C-20.
H.7 A large number of therapeutically active steroids bear a carbonyl group at position 3 and unsaturation across positions 4 and 5. Ring A is often aromatic in estrogens.
J. Tetracyclines
J.1 The tetracycline antibiotics are conveniently named by reference to tetracycline itself, 17, (R = H), which may be defined as (4S,4aS,5aS,6S,12aS)-4-dimethylamino-1,4,4a,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxonaphthacene-2-carboxamide.
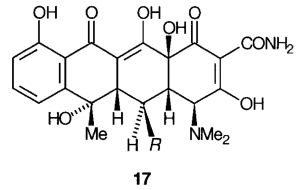
J.2 When analogues of tetracycline are named the stereodescriptors R and S may be subject to change even though the steric configuration usually remains unchanged. For example, in oxytetracycline, the hydroxyl group at position 5 imposes assignment inversions at positions 4a and 5a from S to R although the steric configuration at these positions remains unchanged.

J.3 When fully systematic names based on naphthacene-2-carboxamide are used, the stereodescriptors given in Table III should be used.
K. Tropanes
K.1 Members of the tropane group of substances are conveniently named by references to tropane itself, 18, when the numbering and orientation shown are used. Tropane is defined as (8r)-N-methyl-8-azabicyclo[3.2.1]octane.
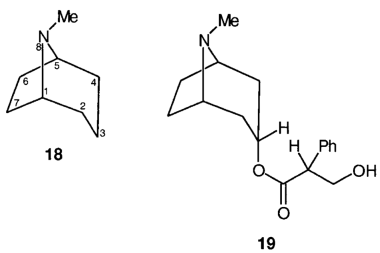
K.2 Atropine, 19, is (1R,3r,5S,8r) tropan-3-yl (RS)-tropate where the term tropate represents 3-hydroxy-2-phenylpropionate.
K.3 In the (–)-series the tropoyl side-chain has the (S) configuration, 20.
K.4 In y-tropane compounds, 21, the configuration at position-3 is designated as ‘3s’.
K.5 In hyoscine and other atropine derivatives bearing a 6,7-epoxy bridge, the configuration is as shown in 22.

L. Xanthines
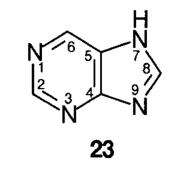
Members of the xanthine group of substances are conveniently named by reference to purine, 23, when the non-systematic numbering shown is used.