The following Supplementary Chapter provides an outline of the mechanism by which monographs are selected and developed for inclusion in the British Pharmacopoeia.
The British Pharmacopoeia Commission will not usually develop monographs for drug substances or excipients. These will usually be elaborated by the European Pharmacopoeia Commission.
The British Pharmacopoeia Commission will consider a monograph for inclusion in the British Pharmacopoeia in the following circumstances:
1. The formulation is widely used (for example: products in the top 500 list of prescribed items; products in the top 100 list of items used in hospitals; Pharmacy (P) and General Sale List (GSL) products that are widely used, taking into account seasonal fluctuations; veterinary products that are widely used or where a need is identified).
2. The innovator product is approaching or past its patent expiry date (monographs will usually only be prepared in the two years preceding patent expiry. However there may be circumstances where it is justified to prepare a monograph at an earlier stage. These will be considered by the British Pharmacopoeia Commission individually).
3. There is a particular need based on the therapeutic category and/or the importance of the material concerned; the latter being particularly relevant to small patient populations.
4. Unlicensed products that are produced to meet particular patient needs (generally “specials” manufacture or extemporaneous preparation) (see also Supplementary Chapter V A).
5. The product/preparation is a widely used traditional herbal medicine.
6. The legal classification of the product has been changed from POM (prescription only medicine) to P or from P to GSL.
7. The product falls within a “family” of product presentations, of which there are already published monographs and/or monographs on the work programme.
8. Drug substances or
excipients which are not on the European Pharmacopoeia work programme, but for which there is a specific UK need.
9. A request is received from the Competent Authority [Medicines and Healthcare Products Regulatory Agency (MHRA)].
10. A request is received from a manufacturer for one of their own products.
11. Support for relevant EC directives.
12. A request is received from official bodies [such as the World Health Organization (WHO)].
13. Other circumstances considered on a case-by-case basis.
It should be noted that compliance with any of the above criteria will not necessarily mean that a monograph will be included in the British Pharmacopoeia. The British Pharmacopoeia Commission may decide not to elaborate a monograph for a number of reasons, including a lack of interest from stakeholders, resource limitations or other circumstances, decided on a case-by-case basis.
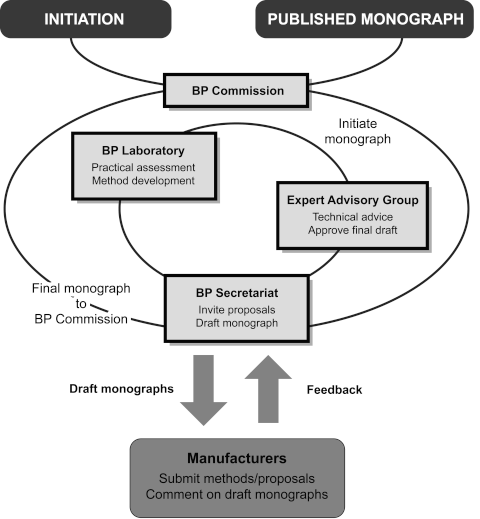
The diagram provides a simplified, schematic representation of the development of a monograph for a medicinal substance or an associated formulated preparation.