SC III F. Validation of Analytical Procedures
Introduction
Validation of an analytical procedure is performed in order to demonstrate that the procedure is suitable for its intended use. Validation is performed in order to show that the result(s) generated by a particular analytical procedure are reliable and accurate.
The principles and practices of validation of analytical procedures are covered by the International Conference on Harmonisation (ICH), are published as guideline Q2(R1), “Validation of Analytical Procedures: Text and Methodology” and are available from www.ich.org. A full discussion of the terms and methodology applicable to validation of analytical procedures is provided in the ICH documents.
Types of procedures to be validated
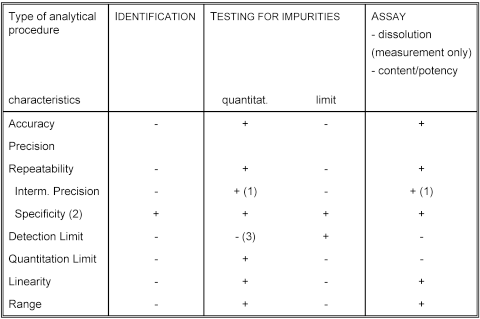
The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose. A tabular summation of the characteristics applicable to identification, control of impurities and assay procedures is included.
- signifies that this characteristic is not normally evaluated
+ signifies that this characteristic is normally evaluated
(1) in cases where reproducibility has been performed, intermediate precision is not needed
(2) lack of specificity of one analytical procedure could be compensated by other supporting analytical procedure(s)
(3) may be needed in some cases
Specificity
Specificity is the ability to assess unequivocally the analyte in the presence of components that may be expected to be present. Typically these might include impurities, degradants, matrix, etc.
Specificity may often be expressed as the degree of bias of test results obtained by analysis of samples containing added impurities, degradation products, related chemical compounds, or placebo ingredients when compared to test results without added substances.
Specificity is usually demonstrated by measuring the response of the sample matrix and any expected or known species (for example excipients, impurities or degradation products). It would normally be expected that no significant response would be obtained that interferes with the measurement of the analyte(s). However it is not always possible that an analytical procedure is specific for a particular analyte. In this instance a combination of two or more analytical procedures may be necessary to achieve the required discrimination.
Linearity
The linearity of an analytical procedure is its ability (within a given range) to obtain test results that are directly proportional to the concentration (amount) of analyte in the sample.
Linearity is usually demonstrated by visual inspection of a plot of signals as a function of analyte concentration or content. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line by the method of least squares. In some cases, to obtain linearity between assays and sample concentrations, the test data may need to be subjected to a mathematical transformation prior to the regression analysis. Data from the regression line itself may be helpful to provide mathematical estimates of the degree of linearity.
The correlation coefficient, y-intercept, slope of the regression line and residual sum of squares should be calculated. A plot of the data should be included. In addition, an analysis of the deviation of the actual data points from the regression line may also be helpful for evaluating linearity.
A minimum of five concentrations is recommended. Other approaches should be justified.
Accuracy
The accuracy of an analytical procedure expresses the closeness of agreement between the value which is accepted either as a conventional true value or an accepted reference value and the value found. This is sometimes termed trueness.
Accuracy should be established across the specified range of the analytical procedure.
Accuracy is usually demonstrated by adding known amounts of analyte(s) to the sample matrix and determining the measured result using the analytical procedure. The recovery of measured against actual amounts is then calculated. Usually a minimum of three determinations at each of three concentrations across the intended range is recommended.
Accuracy may also be demonstrated by the method of standard additions, or by cross-correlation of results with a second, independent, procedure. Accuracy may be inferred once precision, linearity and specificity have been established.
Precision
The precision of an analytical procedure expresses the closeness of agreement (degree of scatter) between a series of measurements obtained from multiple sampling of the same homogeneous sample under the prescribed conditions. Precision may be considered at three levels: repeatability, intermediate precision and reproducibility.
Precision should be investigated using homogeneous, authentic samples. However, if it is not possible to obtain a homogeneous sample it may be investigated using artificially prepared samples or a sample solution.
The precision of an analytical procedure is usually expressed as the variance, standard deviation or coefficient of variation of a series of measurements.
Repeatability (intra-assay precision)
Repeatability expresses the precision under the same operating conditions over a short interval of time. Repeatability is also termed intra-assay precision.
Repeatability is usually demonstrated by repeated measurements of a single sample (e.g. use of the analytical procedure within a laboratory over a short period of time using the same analyst with the same equipment). A minimum of three determinations at each of three concentrations across the intended range, or a minimum of six determinations at the test concentration is recommended.
Intermediate Precision
Intermediate precision expresses within-laboratory variations: different days, different analysts or equipment, etc.
Intermediate precision is usually demonstrated by repeated measurements of the sample used in the repeatability experiment within the same laboratory. Usually the repeatability experiment is repeated on the same sample by a different analyst, on a different day, using different equipment if possible.
Reproducibility
Reproducibility expresses the precision between laboratories (collaborative studies, usually applied to standardisation of methodology).
Reproducibility is usually demonstrated by means of an inter-laboratory trial.
Detection Limit
The detection limit of an analytical procedure is the lowest concentration of analyte in a sample that can be detected but not necessarily quantitated as an exact value.
The detection limit is usually expressed as the concentration of analyte (e.g., percentage, parts per billion) in the sample.
The detection limit is usually demonstrated by measuring low concentrations of the analyte and showing that a response is obtained.
Quantitation Limit
The quantitation limit of an individual analytical procedure is the lowest amount of analyte in a sample which can be quantitatively determined with suitable precision and accuracy. The quantitation limit is a parameter of quantitative assays for low levels of compounds in sample matrices, and is used particularly for the determination of impurities and/or degradation products.
It is usually expressed as the concentration of analyte (e.g. percentage, parts per billion) in the sample.
The quantitation limit is usually demonstrated by measuring low concentrations of the analyte and showing that a repeatable response is obtained.
Robustness
The robustness of an analytical procedure is a measure of its capacity to remain unaffected by small but deliberate variations in method parameters and provides an indication of its reliability during normal usage.
Robustness is usually demonstrated by making small deliberate changes to one of the operating parameters of the method, analysing samples and comparing the results to those obtained using the prescribed method.
Range
The range of an analytical method is the interval between the upper and lower concentration (amounts) of analyte (including these concentrations) for which it has been demonstrated that the analytical procedure has a suitable level of precision, accuracy and linearity.
The specified range is normally derived from linearity studies and depends on the intended application of the procedure. It is established by confirming that the analytical procedure provides an acceptable degree of linearity, accuracy and precision when applied to samples containing amounts of analyte within or at the extremes of the specified range of the analytical procedure.
For assays the range is usually not less than 80 to 120% of the test concentration.
For determination of content uniformity the range is usually not less than 70 to 130% of the test concentration.
For determination of impurities the range is usually not less than the reporting limit of the impurity to 120% of the specification.
For dissolution testing the range is usually +/- 20% over the expected concentrations.
Range is usually demonstrated by confirming that the analytical procedure provides an acceptable degree of linearity, accuracy and precision when applied to samples containing amounts of analyte within or at the extremes of the specified range.
System Suitability Testing
System suitability testing is an integral part of many analytical procedures. The tests are based on the concept that the equipment, electronics, analytical operations and samples to be analyzed constitute an integral system that can be evaluated as such. System suitability test parameters to be established for a particular procedure depend on the type of procedure being validated.
Criteria for assessing the suitability of chromatographic systems are described in the chapter on Chromatographic separation techniques (Appendix III (Ph. Eur. method 2.2.46)). The extent to which adjustments of parameters of the chromatographic system can be made to satisfy the criteria of system suitability are also given in this chapter.