| Entry |
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Azelastine hydrochloride (JP18/USP); Astelin (TN) | ||||||||||
| Product | |||||||||||
| Generic | AZELASTINE HYDROCHLORIDE (A-S Medication Solutions), AZELASTINE HYDROCHLORIDE (Amneal Pharmaceuticals LLC), AZELASTINE HYDROCHLORIDE (AvKARE), AZELASTINE HCL NASAL (Bryant Ranch Prepack), AZELASTINE HCL NASAL (Padagis Israel Pharmaceuticals), AZELASTINE HYDROCHLORIDE (A-S Medication Solutions), AZELASTINE HYDROCHLORIDE (Akorn), AZELASTINE HYDROCHLORIDE (Amneal Pharmaceuticals LLC), AZELASTINE HYDROCHLORIDE (Apotex Corp.), AZELASTINE HYDROCHLORIDE (Apotex Corp.), AZELASTINE HYDROCHLORIDE (Apotex Corp.), AZELASTINE HYDROCHLORIDE (Aurobindo Pharma Limited), AZELASTINE HYDROCHLORIDE (Aurobindo Pharma Limited), AZELASTINE HYDROCHLORIDE (Breckenridge Pharmaceutical), AZELASTINE HYDROCHLORIDE (Bryant Ranch Prepack), AZELASTINE HYDROCHLORIDE (Cadila Healthcare Limited), AZELASTINE HYDROCHLORIDE (Fosun Pharma USA), AZELASTINE HYDROCHLORIDE (Gland Pharma Limited), AZELASTINE HYDROCHLORIDE (NuCare Pharmaceuticals), AZELASTINE HYDROCHLORIDE (NuCare Pharmaceuticals), AZELASTINE HYDROCHLORIDE (Proficient Rx LP), AZELASTINE HYDROCHLORIDE (RPK Pharmaceuticals), AZELASTINE HYDROCHLORIDE (RPK Pharmaceuticals), AZELASTINE HYDROCHLORIDE (Sandoz), AZELASTINE HYDROCHLORIDE (Somerset Therapeutics), AZELASTINE HYDROCHLORIDE (Sun Pharmaceutical Industries), AZELASTINE HYDROCHLORIDE (Sun Pharmaceutical Industries), AZELASTINE HYDROCHLORIDE (West-Ward Pharmaceuticals Corp.), AZELASTINE HYDROCHLORIDE (Zydus Pharmaceuticals (USA)), AZELASTINE HYDROCHLORIDE OPHTHALMIC SOLUTION 0.05% (Alembic Pharmaceuticals) | ||||||||||
| Formula | C22H24ClN3O. HCl | ||||||||||
| Exact mass | 417.1375 | ||||||||||
| Mol weight | 418.3594 | ||||||||||
| Structure | 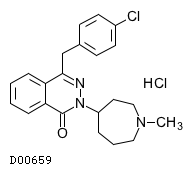 | ||||||||||
| Simcomp | |||||||||||
| Class | Anti-allergic agent DG01557 Histamine receptor antagonist DG01482 Histamine receptor H1 antagonist | ||||||||||
| Remark |
| ||||||||||
| Efficacy | Antiallergic, Antiasthmatic, H1 receptor antagonist | ||||||||||
| Disease | Allergic rhinitis [DS:H01360] | ||||||||||
| Target | HRH1 [HSA:3269] [KO:K04149] | ||||||||||
| Pathway |
| ||||||||||
| Interaction | |||||||||||
| Structure map |
| ||||||||||
| Brite | Anatomical Therapeutic Chemical (ATC) classification [BR:br08303] R RESPIRATORY SYSTEM R01 NASAL PREPARATIONS R01A DECONGESTANTS AND OTHER NASAL PREPARATIONS FOR TOPICAL USE R01AC Antiallergic agents, excl. corticosteroids R01AC03 Azelastine D00659 Azelastine hydrochloride (JP18/USP) <JP/US> R06 ANTIHISTAMINES FOR SYSTEMIC USE R06A ANTIHISTAMINES FOR SYSTEMIC USE R06AX Other antihistamines for systemic use R06AX19 Azelastine D00659 Azelastine hydrochloride (JP18/USP) <JP/US> S SENSORY ORGANS S01 OPHTHALMOLOGICALS S01G DECONGESTANTS AND ANTIALLERGICS S01GX Other antiallergics S01GX07 Azelastine D00659 Azelastine hydrochloride (JP18/USP) <JP/US> USP drug classification [BR:br08302] Ophthalmic Agents Ophthalmic Anti-allergy Agents Antihistamine Azelastine D00659 Azelastine hydrochloride (JP18/USP) Respiratory Tract/Pulmonary Agents Antihistamines Histamine (H1) Receptor Antagonists, Mildly/Non-sedating Azelastine D00659 Azelastine hydrochloride (JP18/USP) Therapeutic category of drugs in Japan [BR:br08301] 4 Agents affecting cellular function 44 Allergic agents 449 Miscellaneous 4490 Miscellaneous D00659 Azelastine hydrochloride (JP18/USP) Classification of Japanese OTC drugs [BR:br08313] Allergic agents 52 Antihistamine based drugs D00659 Azelastine hydrochloride (JP18/USP) 53 Other allergic agents D00659 Azelastine hydrochloride (JP18/USP) Risk category of Japanese OTC drugs [BR:br08312] Second-class OTC drugs Inorganic and organic chemicals Azelastine D00659 Azelastine hydrochloride (JP18/USP) Drug groups [BR:br08330] Anti-allergic agent DG01557 Histamine receptor antagonist DG01482 Histamine receptor H1 antagonist DG01036 Azelastine D00659 Azelastine hydrochloride Drug classes [BR:br08332] Anti-allergic agent DG01482 Histamine receptor H1 antagonist D00659 Azelastine hydrochloride Target-based classification of drugs [BR:br08310] G Protein-coupled receptors Rhodopsin family Histamine HRH1 D00659 Azelastine hydrochloride (JP18/USP) <JP/US> Drugs listed in the Japanese Pharmacopoeia [BR:br08311] Chemicals D00659 Azelastine hydrochloride D00659 Azelastine hydrochloride granules Rx-to-OTC switch list in Japan [br08314.html] D00659 Drug groups [BR:br08330] Anti-allergic agent DG01557 Histamine receptor antagonist DG01482 Histamine receptor H1 antagonist DG01036 Azelastine | ||||||||||
| Other DBs |
| ||||||||||
| KCF data | ATOM 28 1 X Cl 31.8050 -18.2160 2 C1y C 27.4868 -20.1967 3 C1x C 28.5815 -19.3346 4 C1x C 29.9443 -19.6454 5 N1y N 30.5451 -20.8963 6 C1x C 27.4868 -21.6031 7 C1x C 29.9452 -22.1612 8 C1x C 28.5815 -22.4722 9 N4y N 26.2277 -19.5856 10 N5x N 26.2845 -18.1858 11 C8y C 24.9979 -17.4984 12 C8y C 23.7944 -18.2084 13 C8y C 23.8074 -19.6086 14 C8y C 25.0240 -20.2960 15 C8x C 22.5778 -17.5209 16 C8x C 21.3741 -18.2308 17 C8x C 21.3171 -19.6311 18 C8x C 22.6037 -20.3185 19 C1b C 24.9845 -16.0905 20 C8y C 26.2038 -15.4501 21 C8x C 27.4009 -16.2066 22 C8x C 28.7080 -15.5568 23 C8y C 28.6938 -14.1607 24 C8x C 27.4969 -13.4042 25 C8x C 26.1898 -14.0539 26 X Cl 29.9185 -13.5170 27 C1a C 31.9483 -20.9628 28 O5x O 24.9670 -21.6792 BOND 30 1 4 5 1 2 2 6 1 3 5 7 1 4 3 4 1 5 6 8 1 6 2 3 1 7 7 8 1 8 2 9 1 9 9 10 1 10 10 11 2 11 11 12 1 12 12 13 1 13 13 14 1 14 9 14 1 15 12 15 2 16 15 16 1 17 16 17 2 18 17 18 1 19 13 18 2 20 11 19 1 21 19 20 1 22 20 21 2 23 21 22 1 24 22 23 2 25 23 24 1 26 24 25 2 27 20 25 1 28 23 26 1 29 5 27 1 30 14 28 2 |