| Entry |
| ||||||
|---|---|---|---|---|---|---|---|
| Name | Tazobactam and piperacillin (JP18); Piperacillin sodium and tazobactam; Tazocin (TN); Zosyn (TN) | ||||||
| Abbr | TZP | ||||||
| Product | ZOSYN (Wyeth Pharmaceuticals LLC) | ||||||
| Generic | PIPERACILLIN AND TAZOBACTAM (Apotex Corp.), PIPERACILLIN AND TAZOBACTAM (Armas Pharmaceuticals), PIPERACILLIN AND TAZOBACTAM (Armas Pharmaceuticals), PIPERACILLIN AND TAZOBACTAM (Athenex Pharmaceutical Division), PIPERACILLIN AND TAZOBACTAM (Athenex Pharmaceutical Division), PIPERACILLIN AND TAZOBACTAM (BluePoint Laboratories), PIPERACILLIN AND TAZOBACTAM (Fresenius Kabi USA), PIPERACILLIN AND TAZOBACTAM (Fresenius Kabi USA), PIPERACILLIN AND TAZOBACTAM (Fresenius Kabi USA), PIPERACILLIN AND TAZOBACTAM (Fresenius Kabi USA), PIPERACILLIN AND TAZOBACTAM (Fresenius Kabi USA), PIPERACILLIN AND TAZOBACTAM (Fresenius Kabi USA), PIPERACILLIN AND TAZOBACTAM (Fresenius Kabi USA), PIPERACILLIN AND TAZOBACTAM (Hospira), PIPERACILLIN AND TAZOBACTAM (Hospira), PIPERACILLIN AND TAZOBACTAM (Hospira), PIPERACILLIN AND TAZOBACTAM (Piramal Critical Care), PIPERACILLIN AND TAZOBACTAM (Sandoz), PIPERACILLIN AND TAZOBACTAM (Sandoz), PIPERACILLIN AND TAZOBACTAM (Sandoz), PIPERACILLIN AND TAZOBACTAM (Shandong Anxin Pharmaceutical), PIPERACILLIN AND TAZOBACTAM (WG Critical Care), PIPERACILLIN AND TAZOBACTAM (Apollo Pharmaceuticals), PIPERACILLIN AND TAZOBACTAM (Apollo Pharmaceuticals), PIPERACILLIN AND TAZOBACTAM (AuroMedics Pharma LLC), PIPERACILLIN AND TAZOBACTAM (Civica), PIPERACILLIN AND TAZOBACTAM (Civica), PIPERACILLIN AND TAZOBACTAM (Fresenius Kabi USA), PIPERACILLIN AND TAZOBACTAM (Fresenius Kabi USA), PIPERACILLIN AND TAZOBACTAM (Fresenius Kabi USA), PIPERACILLIN AND TAZOBACTAM (Meitheal Pharmaceuticals), PIPERACILLIN AND TAZOBACTAM (Provepharm), PIPERACILLIN AND TAZOBACTAM (Provepharm), PIPERACILLIN AND TAZOBACTAM (Wockhardt USA LLC.), PIPERACILLIN AND TAZOBACTAM (Wockhardt USA LLC.), PIPERACILLIN SODIUM AND TAZOBACTAM SODIUM (Xellia Pharmaceuticals USA LLC), PIPERACILLIN, TAZOBACTAM (Sagent Pharmaceuticals), PIPERACILLIN, TAZOBACTAM (Sagent Pharmaceuticals), PIPERACILLIN, TAZOBACTAM (Sagent Pharmaceuticals), PIPERACILLIN AND TAZOBACTAM (Fresenius Kabi USA), PIPERACILLIN AND TAZOBACTAM (Fresenius Kabi USA), PIPERACILLIN AND TAZOBACTAM (WG Critical Care) | ||||||
| Formula | C23H26N5O7S. C10H12N4O5S. Na | ||||||
| Exact mass | 839.1979 | ||||||
| Mol weight | 839.8277 | ||||||
| Structure | 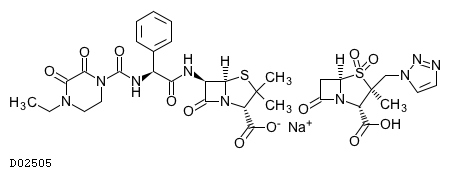 | ||||||
| Component | (Piperacillin sodium [DR:D00466] | Piperacillin hydrate [DR:D02251] | Piperacillin [DR:D08380]), (Tazobactam [DR:D00660] | Tazobactam sodium [DR:D03707]) | ||||||
| Remark |
| ||||||
| Efficacy | Antibacterial | ||||||
| Interaction | |||||||
| Structure map |
| ||||||
| Brite | Anatomical Therapeutic Chemical (ATC) classification [BR:br08303] J ANTIINFECTIVES FOR SYSTEMIC USE J01 ANTIBACTERIALS FOR SYSTEMIC USE J01C BETA-LACTAM ANTIBACTERIALS, PENICILLINS J01CR Combinations of penicillins, incl. beta-lactamase inhibitors J01CR05 Piperacillin and beta-lactamase inhibitor D02505 Tazobactam and piperacillin (JP18) <JP/US> J01R COMBINATIONS OF ANTIBACTERIALS J01RA Combinations of antibacterials J01RA01 Penicillins, combinations with other antibacterials D02505 Tazobactam and piperacillin (JP18) <JP/US> USP drug classification [BR:br08302] Antibacterials Beta-lactam, Penicillins Piperacillin Sodium/ Tazobactam Sodium D02505 Tazobactam and piperacillin (JP18) Therapeutic category of drugs in Japan [BR:br08301] 6 Agents against pathologic organisms and parasites 61 Antibiotics 613 Acting mainly on gram-positive and gram-negative bacteria 6139 Others D02505 Tazobactam and piperacillin (JP18) Antimicrobials [BR:br08307] Antibacterials Cell wall biosynthesis inhibitor, beta-lactam Extended spectrum penicillin D02505 Tazobactam and piperacillin (JP18) <JP/US> Antimicrobials abbreviations [BR:br08327] Antibacterials Cell wall biosynthesis inhibitor, beta-lactam Extended spectrum penicillin D02505 Tazobactam and piperacillin (JP17) Drugs listed in the Japanese Pharmacopoeia [BR:br08311] Chemicals D02505 Tazobactam and piperacillin for injection New drug approvals in Japan [br08318.html] Drugs with new active ingredients D02505 New drug approvals in the USA, Europe and Japan [br08328.html] Approval dates by FDA, EMA and PMDA D02505 | ||||||
| Other DBs |
| ||||||
| KCF data | ATOM 57 1 C1y C 13.8347 -20.3124 2 C5x C 13.8347 -21.7118 3 N1y N 15.2341 -21.7118 4 C1y C 15.2341 -20.3124 5 C1y C 16.5637 -22.1316 6 C1z C 17.4032 -21.0122 7 S2x S 16.5637 -19.8927 8 C1a C 18.3828 -21.9917 9 C1a C 18.3828 -20.0325 10 C6a C 17.0534 -23.5309 11 O6a O 18.4527 -23.5309 #- 12 O6a O 16.2138 -24.6504 13 N1b N 12.6453 -19.6128 14 C5a C 11.4559 -20.3124 15 O5x O 12.6453 -22.4115 16 O5a O 11.4559 -21.7118 17 C1c C 10.1965 -19.6128 18 C8y C 10.1965 -18.2133 19 C8x C 11.4559 -17.5137 20 C8x C 11.4559 -16.1143 21 C8x C 10.1965 -15.4145 22 C8x C 9.0069 -16.1143 23 C8x C 9.0069 -17.5137 24 N1b N 9.0069 -20.3124 25 C5a C 7.8175 -19.6128 26 O5a O 7.8175 -18.2133 27 N1y N 6.5724 -20.3245 28 C5x C 5.3574 -19.6230 29 C5x C 4.1423 -20.3245 30 N1y N 4.1423 -21.7275 31 C1x C 5.3574 -22.4290 32 C1x C 6.5724 -21.7275 33 C1b C 2.9252 -22.4301 34 C1a C 1.7449 -21.7486 35 O5x O 2.9441 -19.6328 36 O5x O 5.3572 -18.2419 37 Z Na 20.0064 -23.5033 #+ 38 C1x C 21.9422 -20.1909 39 C5x C 21.9422 -21.5882 40 N1y N 23.3397 -21.5882 41 C1y C 23.3397 -20.1909 42 C1y C 24.6685 -22.0200 43 C1z C 25.4898 -20.8896 44 S2x S 24.6685 -19.7591 45 C1a C 26.4777 -21.8776 46 C1b C 26.4777 -19.9016 47 C6a C 25.0964 -23.3371 48 O6a O 26.4938 -23.3371 49 O6a O 24.2727 -24.4710 50 O3c O 24.3076 -18.4122 51 O3c O 25.6546 -18.7731 52 N4y N 27.8742 -19.9016 53 C8x C 28.7046 -21.0445 54 C8x C 30.0481 -20.6080 55 N5x N 30.0482 -19.1954 56 N5x N 28.7047 -18.7588 57 O5x O 20.7298 -22.2882 BOND 61 1 1 2 1 2 2 3 1 3 3 4 1 4 1 4 1 5 3 5 1 6 5 6 1 7 6 7 1 8 4 7 1 9 6 8 1 10 6 9 1 11 5 10 1 #Down 12 10 11 1 13 10 12 2 14 1 13 1 #Up 15 13 14 1 16 2 15 2 17 14 16 2 18 14 17 1 19 17 18 1 20 18 19 2 21 19 20 1 22 20 21 2 23 21 22 1 24 22 23 2 25 18 23 1 26 24 25 1 27 17 24 1 #Up 28 25 26 2 29 25 27 1 30 27 28 1 31 28 29 1 32 29 30 1 33 30 31 1 34 31 32 1 35 27 32 1 36 30 33 1 37 33 34 1 38 29 35 2 39 28 36 2 40 38 39 1 41 39 40 1 42 40 41 1 43 38 41 1 44 40 42 1 45 42 43 1 46 43 44 1 47 41 44 1 48 43 45 1 #Down 49 43 46 1 50 42 47 1 #Down 51 47 48 1 52 47 49 2 53 44 50 2 54 44 51 2 55 46 52 1 56 52 53 1 57 53 54 2 58 54 55 1 59 55 56 2 60 52 56 1 61 39 57 2 |