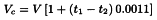Perchloric Acid VS
HClO4 = 100.5
For a 0.1m solution Place 8.5 mL of perchloric acid in a volumetric flask containing about 900 mL of glacial acetic acid and mix. Add 30 mL of acetic anhydride, dilute to 1000.0 mL with glacial acetic acid, mix and allow to stand for 24 h. Determine the water content (2.5.12) without addition of methanol and, if necessary, adjust the water content to 0.1-0.2 per cent by adding either acetic anhydride or water. Allow to stand for 24 h.
Standardisation. Dissolve 0.170 g of potassium hydrogen phthalate in 50 mL of anhydrous acetic acid, warming gently if necessary. Allow to cool protected from air, and titrate with the perchloric acid solution, determining the end-point potentiometrically (2.2.20) or using 0.05 mL of crystal violet solution as indicator. Note the temperature of the perchloric acid solution at the time of the titration. If the temperature at which an assay is carried out is different from that at which the 0.1 M perchloric acid has been standardised, the volume used in the assay becomes:
| t1 | = | temperature during standardisation, |
| t2 | = | temperature during the assay, |
| Vc | = | corrected volume, |
| V | = | observed volume. |
1 mL of 0.1 M perchloric acid is equivalent to 20.42 mg of C8H5KO4.
Dilution Use anhydrous acetic acid.
For a 0.02m solution Dilute 20.0 mL of 0.1m perchloric acid to 100.0 mL with anhydrous acetic acid.
Other strengths of perchloric acid should be prepared by diluting 0.1m perchloric acid VS appropriately with anhydrous acetic acid.