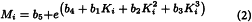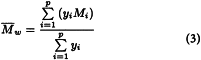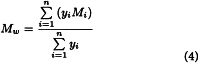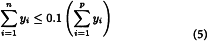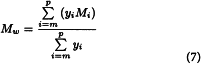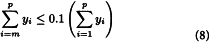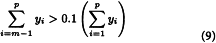Appendix III C. Size-exclusion Chromatography
Size-exclusion chromatography is a chromatographic technique which separates molecules in solution according to their size. With organic mobile phases, the technique is known as gel-permeation chromatography and with aqueous mobile phases, the term gel-filtration chromatography has been used. The sample is introduced into a column, which is filled with a gel or a porous particle packing material, and is carried by the mobile phase through the column. The size separation takes place by repeated exchange of the solute molecules between the solvent of the mobile phase and the same solvent in the stagnant liquid phase (stationary phase) within the pores of the packing material. The pore-size range of the packing material determines the molecular-size range within which separation can occur.
Molecules small enough to penetrate all the pore spaces elute at the total permeation volume (Vt). On the other hand, molecules apparently larger than the maximum pore size of the packing material migrate along the column only through the spaces between the particles of the packing material without being retained and elute at the exclusion volume (V0 void volume). Separation according to molecular size occurs between the exclusion volume and the total permeation volume, with useful separation usually occurring in the first two thirds of this range.
Apparatus The apparatus consists essentially of a chromatographic column of varying length and internal diameter (Ø), if necessary temperature-controlled, packed with a separation material that is capable of fractionation in the appropriate range of molecular sizes and through which the eluent is passed at a constant rate. One end of the column is usually fitted with a suitable device for applying the sample such as a flow adapter, a syringe through a septum or an injection valve and may also be connected to a suitable pump for controlling the flow of the eluent. Alternatively the sample may be applied directly to the drained bed surface or, where the sample is denser than the eluent, it may be layered beneath the eluent. The outlet of the column is usually connected to a suitable detector fitted with an automatic recorder which enables the monitoring of the relative concentrations of separated components of the sample. Detectors are usually based on photometric, refractometric or luminescent properties. An automatic fraction collector may be attached, if necessary.
The packing material may be a soft support such as a swollen gel or a rigid support composed of a material such as glass, silica or a solvent-compatible, cross-linked organic polymer. Rigid supports usually require pressurised systems giving faster separations. The mobile phase is chosen according to sample type, separation medium and method of detection. Before carrying out the separation, the packing material is treated, and the column is packed, as described in the monograph, or according to the manufacturer’s instructions.
Criteria for assessing the suitability of the system are described in the chapter on Chromatographic separation techniques (2.2.46). The extent to which adjustments of parameters of the chromatographic system can be made to satisfy the criteria of system suitability are also given in this chapter.
Determination of relative component composition of mixtures
Carry out the separation as stated in the monograph. If possible, monitor the elution of the components continuously and measure the corresponding peak areas. If the sample is monitored by a physico-chemical property to which all the components of interest exhibit equivalent responses (for example if they have the same specific absorbance), calculate the relative amount of each component by dividing the respective peak area by the sum of the peak areas of all the components of interest. If the responses to the property used for detection of the components of interest are not equivalent, calculate the content by means of calibration curves obtained with the calibration standards prescribed in the monograph.
Determination of molecular masses
Size-exclusion chromatography may be used to determine molecular masses by comparison with appropriate calibration standards specified in the monograph. The retention volumes of the calibration standards may be plotted against the logarithm of their molecular masses. The plot usually approximates a straight line within the exclusion and total permeation limits for the separation medium used. From the calibration curve, molecular masses may be estimated. The molecular-mass calibration is valid only for the particular macromolecular solute/solvent system used under the specified experimental conditions.
Determination of molecular size distribution of polymers
Size-exclusion chromatography may be used to determine the distribution of the molecular size of polymers. However, sample comparison may be valid only for results obtained under the same experimental conditions. The reference substances used for the calibration and the methods for determination of the distribution of molecular sizes of polymers are specified in the monograph.
Molecular Mass Distribution in Dextrans
Examine by size-exclusion chromatography (2.2.30).
Test solution Dissolve 0.200 g of the substance to be examined in the mobile phase and dilute to 10 mL with the mobile phase.
Marker solution Dissolve 5 mg of glucose R and 2 mg of dextran V0 CRS in 1 mL of the mobile phase.
Calibration solutions Dissolve separately in 1 mL of the mobile phase 15 mg of dextran 4 for calibration CRS, 15 mg of dextran 10 for calibration CRS, 20 mg of dextran 40 for calibration CRS, 20 mg of dextran 70 for calibration CRS and 20 mg of dextran 250 for calibration CRS.
System suitability solution Dissolve either 20 mg of dextran 40 for performance test CRS (for dextran 40) or 20 mg of dextran 60/70 for performance test CRS (for dextran 60 and dextran 70) in 1 mL of the mobile phase.
The chromatographic procedure may be carried out using:
maintaining the system at a constant temperature (± 0.1 °C).
CALIBRATION OF THE CHROMATOGRAPHIC SYSTEM
Carry out replicate injections of the chosen volume of the marker solution. The chromatogram shows 2 peaks, the first of which corresponds to dextran V0 CRS and the second of which corresponds to glucose R. From the elution volume of the peak corresponding to dextran V0, calculate the void volume V0 and from the peak corresponding to dextrose, calculate the total volume Vt.
Inject the chosen volume of each of the calibration solutions. Draw carefully the baseline of each of the chromatograms. Divide each chromatogram into p (at least 60) equal vertical sections (corresponding to equal elution volumes). In each section i, corresponding to an elution volume Vi measure the height (yi) of the chromatogram line above the baseline and calculate the coefficient of distribution Ki using the expression:
V0 | = | |
Vt | = | total volume of the column, determined using the peak corresponding to glucose in the chromatogram obtained with the marker solution, |
Vi | = | elution volume of section i in the chromatogram obtained with each of the calibration solutions. |
Carry out the calibration using either of the following methods.
Calibration by plotting of the curve
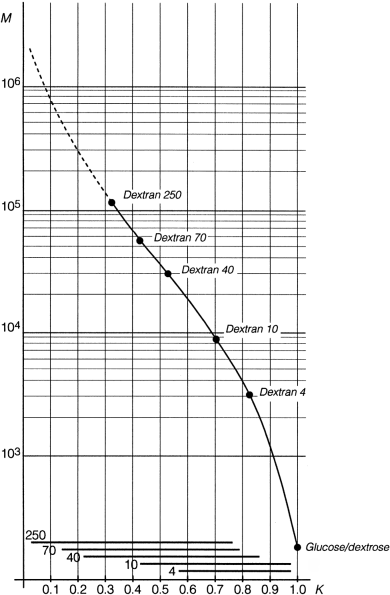
For each of the dextrans for calibration calculate the coefficient of distribution Kmax corresponding to the maximum height of the chromatographic line, using expression (1). Plot on semilogarithmic paper the values of Kmax (on the x-axis) against the declared molecular mass at the maximum height of the chromatographic line (Mmax) of each of the dextrans for calibration and glucose. Draw a first calibration curve through the points obtained, extrapolating it from the point Kmax obtained with dextran 250 for calibration CRS to the lowest K value obtained for this CRS (Figure 2.2.39.-1). Using this first calibration curve, transform, for each chromatogram, all Ki values into the corresponding molecular mass Mi, thus obtaining the molecular mass distribution. Calculate for each dextran for calibration the average molecular mass Mw using equation (3) below. If the calculated values for Mw do not differ by more than 5 per cent from those declared for each of the dextrans for calibration and the mean difference is within ± 3 per cent, the calibration curve is approved. If not, move the calibration curve along the y-axis and repeat the procedure above until the calculated and the declared values for Mw do not differ by more than 5 per cent.
Calibration by calculation of the curve
Calculate from equations (2) and (3) below, using a suitable method1, values for b1, b2, b3, b4 and b5 that give values of Mw within 5 per cent of the declared values of each of the dextrans for calibration and 180 ± 2 for glucose:
p | = | number of sections dividing the chromatograms, |
yi | = | height of the chromatographic line above the baseline in section i, |
Mi | = | molecular mass in section i. |
SYSTEM SUITABILITY
Inject the chosen volume of the appropriate system suitability solution.
Average molecular mass of dextran for performance test CRS
Calculate the average molecular mass Mw as indicated under Calibration of the chromatographic system, using either the plotted calibration curve or the values obtained above for b1, b2, b3, b4 and b5. The test is not valid unless Mw is:
Average molecular mass of the 10 per cent high-fraction dextran
Calculate Mw for the 10 per cent high-fraction dextran eluted through section n using the equation:
in which n is defined by the expressions:
p | = | number of sections dividing the chromatograms, |
yi | = | height of the chromatographic line above the baseline in section i, |
Mi | = | molecular mass in section i. |
The test is not valid unless Mw of the 10 per cent high fraction dextran is:
Average molecular mass of the 10 per cent low-fraction dextran
Calculate Mw for the 10 per cent low-fraction dextran eluted in and after section m using the expression:
in which m is defined by the expressions:
p | = | number of sections dividing the chromatograms, |
yi | = | height of the chromatographic line above the baseline in section i, |
Mi | = | molecular mass in section i. |
The test is not valid unless Mw of the 10 per cent low-fraction dextran is:
MOLECULAR MASS DISTRIBUTION OF THE DEXTRAN TO BE ANALYSED
Inject the chosen volume of the test solution and calculate Mw of the total molecular mass distribution, Mw of the 10 per cent high-fraction dextran and Mw of the 10 per cent low-fraction dextran as indicated under System suitability.