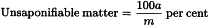Appendix X H. Unsaponifiable Matter
The unsaponifiable matter is the percentage content, w/w, of material not volatile at 100° to 105° that is obtained by extraction with an organic solvent from the saponified substance being examined.
Use Method I unless otherwise specified in the monograph. Use ungreased ground-glass glassware for each method.
Method I
To 2.0 to 2.5 g of the substance being examined in a 250-mL flask add 25 mL of 0.5m ethanolic potassium hydroxide and boil under a reflux condenser in a water-bath for 1 hour, swirling the contents frequently. Wash the contents of the flask into a separating funnel with the aid of 50 mL of water and, while the liquid is still slightly warm, extract by shaking vigorously with three 50-mL quantities of peroxide-free ether, rinsing the flask with the first quantity of ether. Mix the ether solutions in a separating funnel containing 20 mL of water. (If the ether solutions contain solid suspended matter, filter them into the separating funnel through a fat-free filter paper and wash the filter paper with peroxide-free ether.) Gently rotate the separating funnel for a few minutes without violent shaking, allow the liquids to separate and discard the aqueous layer. Wash the ether solution by shaking vigorously with two 20-mL quantities of water and then treat with three 20-mL quantities of 0.5m potassium hydroxide, shaking vigorously on each occasion, each treatment being followed by washing with 20 mL of water. Finally wash with successive 20-mL quantities of water until the aqueous layer is no longer alkaline to phenolphthalein solution R1. Transfer the ether extract to a weighed flask, rinsing the separating funnel with peroxide-free ether, distil the ether and add 3 mL of acetone to the flask. With the aid of a gentle current of air, remove the solvent completely from the flask, which is almost entirely immersed in boiling water and preferably held obliquely and rotated. Dry to constant weight at a temperature not exceeding 80° and dissolve the contents of the flask in 10 mL of freshly boiled ethanol (96%), previously neutralised to phenolphthalein solution R1. Titrate with 0.1m ethanolic sodium hydroxide VS using phenolphthalein solution R1 as indicator.
If the volume of 0.1m ethanolic sodium hydroxide VS required does not exceed 0.1 mL, the amount of residue weighed is to be taken as the unsaponifiable matter. Calculate the unsaponifiable matter as a percentage of the substance being examined.
If the volume of 0.1m ethanolic sodium hydroxide VS required exceeds 0.1 mL, the amount of residue weighed cannot be taken as the unsaponifiable matter and the test must be repeated.
Method II
The term “unsaponifiable matter” is applied to the substances non-volatile at 100-105 °C obtained by extraction with an organic solvent from the substance to be examined after it has been saponified. The result is calculated as per cent m/m.
Use ungreased ground-glass glassware.
Introduce the prescribed quantity of the substance to be examined (m g) into a 250 mL flask fitted with a reflux condenser. Add 50 mL of 2 M alcoholic potassium hydroxide R and heat on a water-bath for 1 h, swirling frequently. Cool to a temperature below 25 °C and transfer the contents of the flask to a separating funnel with the aid of 100 mL of water R. Shake the liquid carefully with 3 quantities, each of 100 mL, of peroxide-free ether R. Combine the ether layers in another separating funnel containing 40 mL of water R, shake gently for a few minutes, allow to separate and reject the aqueous phase. Wash the ether phase with 2 quantities, each of 40 mL, of water R then wash successively with 40 mL of a 30 g/L solution of potassium hydroxide R and 40 mL of water R; repeat this procedure 3 times. Wash the ether phase several times, each with 40 mL of water R, until the aqueous phase is no longer alkaline to phenolphthalein. Transfer the ether phase to a tared flask, washing the separating funnel with peroxide-free ether R.
Distil off the ether with suitable precautions and add 6 mL of acetone R to the residue. Carefully remove the solvent in a current of air. Dry to constant mass at 100-105 °C. Allow to cool in a desiccator and weigh (a g).
Dissolve the residue in 20 mL of alcohol R, previously neutralised to phenolphthalein solution R and titrate with 0.1 M ethanolic sodium hydroxide. If the volume of 0.1 M ethanolic sodium hydroxide used is greater than 0.2 mL, the separation of the layers has been incomplete; the residue weighed cannot be considered as “unsaponifiable matter”. In case of doubt, the test must be repeated.