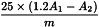Appendix X O. Anisidine Value
The anisidine value is defined as 100 times the optical density measured in a 1 cm cell of a solution containing 1 g of the substance to be examined in 100 mL of a mixture of solvents and reagents according to the following method.
Carry out the operations as rapidly as possible, avoiding exposure to actinic light.
Test solution (a). Dissolve 0.500 g of the substance to be examined in trimethylpentane R and dilute to 25.0 mL with the same solvent.
Test solution (b). To 5.0 mL of test solution (a) add 1.0 mL of a 2.5 g/L solution of p-anisidine R in glacial acetic acid R, shake and store protected from light.
Reference solution. To 5.0 mL of trimethylpentane R add 1.0 mL of a 2.5 g/L solution of p-anisidine R in glacial acetic acid R, shake and store protected from light.
Measure the absorbance (2.2.25) of test solution (a) at the maximum at 350 nm using trimethylpentane R as the compensation liquid. Measure the absorbance of test solution (b) at 350 nm exactly 10 min after its preparation, using the reference solution as the compensation liquid.
Calculate the anisidine value from the expression:
| A1 | = | absorbance of test solution (b) at 350 nm, |
| A2 | = | absorbance of test solution (a) at 350 nm, |
| m | = | mass of the substance to be examined in test solution (a), in grams. |