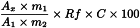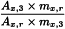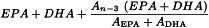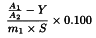Appendix X P. Oils Rich in Omega-3-acids
1. Composition of Fatty Acids
The assay may be used for quantitative determination of the eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and total omega-3-acids content in products from fish oil containing omega-3 acids in different concentrations. The method is applicable to triglycerides or ethyl esters. The former includes fish oils/fish-liver oils and omega-3-concentrates in triglyceride form. The results are expressed as triglycerides or ethyl esters, respectively.
EPA and DHA
Gas chromatography (2.2.28). Carry out the operations as rapidly as possible, avoiding exposure to actinic light, oxidising agents, oxidation catalysts (for example, copper and iron) and air.
The assay is carried out on the methyl esters (after derivatisation of triglycerides - see step B below) or ethyl esters of (all-Z)-eicosa-5,8,11,14,17-pentaenoic acid (EPA; 20:5 n-3) and (all-Z)-docosa-4,7,10,13,16,19-hexaenoic acid (DHA; 22:6 n-3) in the substance to be examined.
Internal standard methyl tricosanoate R.
Test solutions Prepare all test solutions in duplicate.
Step A
Step B
Introduce 2.0 mL of test solutions (a) and (b) obtained in step A into separate quartz tubes and evaporate the solvent with a gentle current of nitrogen R. Add 1.5 mL of a 20 g/L solution of sodium hydroxide R in methanol R, cover with nitrogen R, cap tightly with a polytetrafluoroethylene-lined cap, mix and heat on a water-bath for 7 min. Allow to cool. Add 2 mL of boron trichloride-methanol solution R, cover with nitrogen R, cap tightly, mix and heat on a water-bath for 30 min. Cool to 40-50 °C, add 1 mL of trimethylpentane R, cap and shake vigorously for at least 30 s. Immediately add 5 mL of a saturated sodium chloride solution R, cover with nitrogen R, cap and shake thoroughly for at least 15 s. Transfer the upper layer to a separate tube. Shake the methanol layer once more with 1 mL of trimethylpentane R. Wash the combined trimethylpentane extracts with 2 quantities, each of 1 mL, of water R and dry over anhydrous sodium sulfate R.
Reference solutions Prepare reference solutions (a1) and (a2) in duplicate; reference solution (c) only has to be prepared for triglycerides, and only if tetracos-15-enoic acid methyl ester is not clearly observed in the chromatogram obtained with test solution (a).
For ethyl ester samples, reference solutions (a1) and (a2) are ready for analysis. For analysis of triglycerides, continue with step B in the same manner as for test solutions (a) and (b).
Carrier gas hydrogen for chromatography R or helium for chromatography R.
Flow rate 1 mL/min.
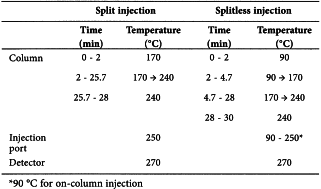
Split ratio 1:200, alternatively splitless with temperature control (sample solutions need to be diluted 1/200 with a 50 mg/L solution of butylhydroxytoluene R in trimethylpentane R before injection).
If necessary, adapt the split ratio and/or sample dilution to obtain a symmetry factor of 0.8-1.5 for the peaks due to the methyl or ethyl esters of eicosapentaenoic acid and docosahexaenoic acid, at the same time observing that for test solution (b) any peaks due to the corresponding esters of linolenic acid (C18:3; n-3), stearidonic acid (C18:4; n-3), eicosatetraenoic acid (C20:4; n-3), heneicosapentaenoic acid (C21:5; n-3), and docosapentaenoic acid (C22:5; n-3) are clearly detectable.
If necessary, adapt the split ratio and/or sample dilution to obtain a symmetry factor of 0.8-1.5 for the peaks due to the components of reference solution (b).
Detection Flame ionisation.
Injection 1 µL.
Calculate the percentage content of EPA and DHA using the following expression and taking into account the assigned value of the reference substances:
Rf | = | response factor for EPA and DHA as given by the expression: |
m1 | = | mass of the internal standard in test solution (a), in milligrams; |
m2 | = | mass of the sample to be examined in test solution (a), in milligrams; |
mx,3 | = | mass of the internal standard in reference solution (a1) (EPA determination), or in reference solution (a2) (DHA determination), in milligrams; |
mx,r | = | mass of eicosapentaenoic acid ethyl ester CRS in reference solution (a1) or docosahexaenoic acid ethyl ester CRS in reference solution (a2), in milligrams; |
Ax | = | area of the peak due to eicosapentaenoic acid ester or docosahexaenoic acid ester in the chromatogram obtained with test solution (a); |
Ax,r | = | area of the peak due to eicosapentaenoic acid ester in the chromatogram obtained with reference solution (a1) or docosahexaenoic acid ester in the chromatogram obtained with reference solution (a2); |
A1 | = | area of the peak due to the internal standard in the chromatogram obtained with test solution (a); |
Ax,3 | = | area of the peak due to the internal standard in the chromatogram obtained with reference solution (a1) (EPA determination) or with reference solution (a2) (DHA determination); |
C | = | conversion factor between ethyl ester and triglycerides: |
TOTAL OMEGA-3 ACIDS
From the assay for EPA and DHA, calculate the percentage content of the total omega-3 acids using the following expression and identifying the peaks from the chromatograms:
EPA | = | percentage content of EPA; |
DHA | = | percentage content of DHA; |
An-3 | = | sum of the areas of the peaks due to linolenic acid (C18:3; n-3), stearidonic acid (C18:4; n-3), eicosatetraenoic acid (C20:4; n-3), heneicosapentaenoic acid (C21:5; n-3), and docosapentaenoic acid (C22:5; n-3) in the chromatogram obtained with test solution (b); |
AEPA | = | area of the peak due to EPA ester in the chromatogram obtained with test solution (b); |
ADHA | = | area of the peak due to DHA ester in the chromatogram obtained with test solution (b). |
2. Total Cholesterol in Oils Rich in Omega-3-Acids
Internal standard stock solution Dissolve 0.15 g of (5α)-cholestane R in heptane R and dilute to 50.0 mL with the same solvent. The solution may be stored in a deep-freeze for up to 6 months.
Internal standard working solution. Prepare the solution immediately before use Dilute 1.0 mL of the internal standard stock solution to 10.0 mL with heptane R.
Cholesterol stock solution Dissolve 50.0 mg of cholesterol R in heptane R and dilute to 100.0 mL with the same solvent. The solution may be stored in a deep-freeze for up to 6 months.
Cholesterol working solution. Prepare the solution immediately before use Dilute 1.0 mL of the cholesterol stock solution to 10.0 mL with heptane R.
Cholesterol and α-tocopherol stock solution Dissolve 50.0 mg of cholesterol R and 50.0 mg of α-tocopherol R in heptane R and dilute to 100.0 mL with the same solvent. The solution may be stored at room temperature for up to 3 months.
Reference solution. Prepare the solution on the day of use Dilute 1.0 mL of the cholesterol and α-tocopherol stock solution to 100.0 mL with heptane R.
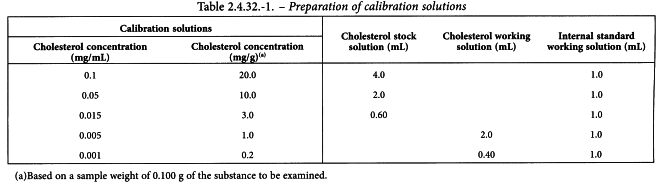
Calibration solutions See Table 2.4.32.-1. Prepare the solutions on the day of use. Dilute each solution to 20.0 mL with a 10 per cent V/V solution of ethyl acetate R in heptane R.
For high levels of cholesterol (3.0-20.0 mg/g), use all 5 calibration solutions.
For low levels of cholesterol (0.2-3.0 mg/g), use the following calibration solutions: 0.2 mg/g, 1.0 mg/g and 3.0 mg/g.
Test solution Weigh 0.100 g of the substance to be examined into a 15 mL quartz tube (for fish oils and cod-liver oils, shake the oil to be examined vigorously in a suitable container, allow to stand for 10-15 min and while maintaining the container upright, remove a sample from the middle layer of the oil for weighing). Add 1.0 mL of the internal standard working solution. Evaporate the solvent on a heating block at 50 °C under a gentle stream of nitrogen R. Add 0.5 mL of a 50 per cent m/m solution of potassium hydroxide R and 3.0 mL of ethanol (96 per cent) R. Fill the tube with nitrogen R, cap and homogenise. Heat on the heating block at 100 °C for 1 h. Cool for about 10 min, add 6.0 mL of distilled water R and homogenise. Condition a 20 mL solid phase extraction (SPE) column containing 1 g of end-capped octadecylsilyl silica gel for chromatography R (particles with a diameter of 55 µm and a pore size of 7 nm) with 5 mL of a 50 per cent V/V solution of ethanol (96 per cent) R in distilled water R. Transfer 5.0 mL of the saponified sample to the SPE column ensuring that the column does not dry out. Wash the column with 5.0 mL of a 50 per cent V/V solution of ethanol (96 per cent) R in distilled water R. Elute the column using 20.0 mL of a 10 per cent V/V solution of ethyl acetate R in heptane R. Collect the eluate and use it as the test solution.
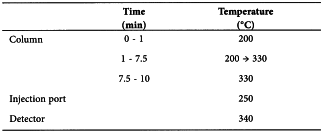
Carrier gas helium for chromatography R.
Pressure 48 kPa (corresponding to a flow rate of about 0.6 mL/min at 200 °C and about 0.4 mL/min at 330 °C).
Split ratio 1:5.
Prior to analysis, heat the column at 340 °C for at least 30 min using the indicated pressure.
Detection Flame ionisation.
Injection 1 µL.
Retention time (5α)-cholestane = about 7.5 min; cholesterol = about 9 min.
Plot the calibration curve. The x-axis represents the nominal concentration of cholesterol in milligrams per gram of the substance to be examined in each calibration solution. The y-axis represents the ratio of the area of the peak due to cholesterol to the area of the peak due to (5α)-cholestane in the chromatogram obtained with each calibration solution. Calculate the slope (S) and the intercept with the y-axis (Y).
Calculate the content of total cholesterol, expressed in milligrams of cholesterol per gram of substance to be examined, using the following expression:
A1 | = | area of the peak due to cholesterol in the chromatogram obtained with the test solution; |
A2 | = | area of the peak due to (5α)-cholestane in the chromatogram obtained with the test solution; |
m1 | = | mass of the substance to be examined in the test solution, in grams; |
Y | = | intercept of the calibration curve with the y-axis; |
S | = | slope of the calibration curve, in grams per milligram. |