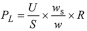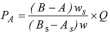Appendix XIV I. Assay of Pancreatin
The free protease, lipase and amylase activities of pancreatin are determined by the following methods.
Standard preparation and units
The Standard Preparation is the appropriate FIP Standard which has been adopted as an official preparation by the European Pharmacopoeia Commission and is available as pancreas powder (protease) EPBRP or pancreas powder (amylase and lipase) EPBRP as appropriate.
The Unit of protease activity is contained in that amount of the Standard Preparation that, under the conditions of the assay, hydrolyses casein at an initial rate such that there is liberated per minute an amount of peptides not precipitated by trichloroacetic acid that gives the same absorbance at 275 nm as one micromole of tyrosine. The Unit of lipase activity is contained in that amount of the Standard Preparation that, under the conditions of the assay, liberates one micro-equivalent of acid per minute at pH 9.0 and 37°. The Unit of amylase activity is contained in that amount of the standard preparation that, under the conditions of the assay, decomposes starch at an initial rate such that one micro-equivalent of glycosidic linkage is hydrolysed per minute.
1. Free protease activity
Method
Solution of the standard preparation Triturate for 5 minutes a quantity of the Standard Preparation containing approximately 100 Units of protease activity with 25 mL of calcium chloride solution cooled to 5°. Dilute to 100 mL with the cooled calcium chloride solution and then dilute a sufficient quantity of the resulting suspension to 100 mL with borate buffer pH 7.5, cooled to 5°, so that 1 mL of the final solution contains 0.065 Units of protease activity.
Solution of the substance being examined Triturate for 5 minutes a quantity of the substance being examined containing approximately 100 Units of protease activity with 25 mL of calcium chloride solution cooled to 5°. Dilute to 100 mL with the cooled calcium chloride solution and then dilute a sufficient quantity of the resulting suspension to 100 mL with borate buffer pH 7.5, cooled to 5°, so that the estimated free protease activity corresponds approximately to the activity of the solution of the Standard Preparation.
Label 16 test tubes with the following identification in duplicate; S1, S2, S3, S1B, S2B, S3B, U and UB. To tubes S1 and S1B add 2.0 mL and to tubes S2, S2B, U and UB, 1.0 mL of borate buffer pH 7.5. Then to tubes S1 and S1B add 1.0 mL, to tubes S2 and S2B, 2.0 mL and to tubes S3 and S3B, 3.0 mL of the solution of the Standard Preparation. Add 2.0 mL of the solution of the substance being examined to tubes U and UB. To each of the control tubes (S1B, S2B, S3B and UB) add 5.0 mL of a 5% w/v solution of trichloroacetic acid and mix. Place a stirring rod in each tube and warm to, and maintain at, 35° in a water bath. Add 5.0 mL of concentrated casein substrate to each of the control tubes and mix.
At accurately timed intervals add 5.0 mL of concentrated casein substrate, previously warmed to 35°, to tubes S1, S2, S3 and U and mix immediately. After exactly 30 minutes, in the same order, stop the reaction in tubes S1, S2, S3 and U by adding 5.0 mL of a 5% w/v solution of trichloroacetic acid and mix thoroughly. Remove all the test tubes from the water bath and allow to stand at room temperature for 20 minutes. Filter the contents of the tubes through suitable filter paper1, collect the filtrates and refilter through the same paper. The filtrates must be free from haze.
Measure the absorbances of the filtrates at the maximum at 275 nm, Appendix II B, using in the reference cell a mixture of 6.0 mL of borate buffer pH 7.5 and 5.0 mL of the 5% w/v solution of trichloroacetic acid that has been filtered in the same way. Correct the mean absorbances of the filtrates from tubes S1, S2 and S3 by subtracting the mean absorbances of the filtrates from the corresponding control tubes S1B, S2B and S3B.
Prepare a reference curve by plotting the mean corrected absorbances against the potency of the dilution of the solution of the Standard Preparation used. Calculate the corrected mean absorbance of the substance being examined by subtracting the mean absorbance of the filtrates from tubes UB from that of the filtrates from tubes U. Using the corrected mean absorbance, determine the potency of the solution of the substance being examined from the reference curve and calculate the free protease activity per mg of the substance being examined by taking into account the dilution factors.
The test is not valid unless the corrected absorbances are between 0.15 and 0.60.
2. Lipase activity
Apparatus
Use a reaction vessel of about 50 mL capacity fitted with a device that will maintain a temperature of 36.5° to 37.5°, a magnetic stirrer and a lid with holes for the insertion of electrodes, the tip of a burette, a tube for the admission of nitrogen and the introduction of reagents. An automatic or manual titration apparatus may be used. In the latter case, the burette is graduated in 5-µL divisions and the pH meter is provided with a wide reading scale and glass and calomel electrodes. After each test the reaction vessel is evacuated by suction and washed several times with water, the washings being removed each time by suction.
Method
Carry out the assay under nitrogen. In a small mortar cooled to 0° to 4° triturate carefully an amount of the substance being examined containing approximately 2500 Units of lipase activity with 1 mL of cooled lipase solvent until a very fine suspension is obtained (about 10 minutes). Dilute with cooled lipase solvent, transfer quantitatively to a graduated flask and dilute to 100.0 mL with the cooled solvent; use immediately.
Transfer 29.5 mL of olive oil substrate emulsion to the assembled reaction vessel equilibrated at 36.5° to 37.5° and adjust the pH to 9.2 with 0.1m sodium hydroxide. Add about 0.5 mL of the suspension of the preparation being examined and record the time at which the pH reaches 9.0. Add continuously from a micrometer syringe sufficient 0.1m sodium hydroxide VS to maintain the pH at 9.0. Record the volume of 0.1m sodium hydroxide VS consumed at 1-minute intervals for 5 minutes. Discounting the first reading, calculate the mean rate of alkali consumption U. If necessary, dilute with sufficient lipase solvent to produce an average alkali consumption of 0.08 to 0.16 mL of 0.1m sodium hydroxide VS per minute. Repeat the procedure using the Standard Preparation in place of the substance being examined and calculate the mean rate of alkali consumption, S. Calculate the potency (PL) of the substance being examined in Units per mg from the expression:
Where | U | = | the mean volume in mL of 0.1m sodium hydroxide VS used per minute in the titration of the substance being examined, |
S | = | the mean volume in mL of 0.1m sodium hydroxide VS used per minute in the titration of the Standard Preparation, | |
w | = | weight in mg of the substance being examined, | |
ws | = | weight in mg of the Standard Preparation, | |
R | = | potency of the Standard Preparation in Units per mg. |
Calculate the potency of the preparation being examined using the average of three separate titrations for both the substance being examined and the Standard Preparation.
3. Amylase activity
Method
Triturate an amount of the preparation being examined containing approximately 1500 Units of amylase activity with 60 mL of 0.2m mixed phosphate buffer pH 6.8 for 15 minutes and add sufficient 0.2m mixed phosphate buffer pH 6.8 to produce 100 mL. To a stoppered tube (200 mm × 22 mm) add 25.0 mL of starch substrate, 10.0 mL of 0.2m mixed phosphate buffer pH 6.8 and 1.0 mL of 0.2m sodium chloride. Stopper the tube, mix the contents and place in a water bath at 24.9° to 25.1°. When the temperature of the mixture has reached 25° add 1.0 mL of the solution of the substance being examined and record the time of addition. Mix thoroughly and replace in the water bath. After exactly 10 minutes add 2 mL of 1m hydrochloric acid to stop the reaction. Transfer the contents of the tube to a stoppered 300 mL flask. While shaking continuously add 10.0 mL of 0.05M iodine VS followed immediately by 45 mL of 0.1m sodium hydroxide. Allow to stand in the dark at a temperature of 15° to 25° for 15 minutes. Add 4 mL of a mixture of 1 volume of sulfuric acid and 4 volumes of water and titrate with 0.1m sodium thiosulfate VS. Repeat the procedure but add the 2 mL of 1m hydrochloric acid before the addition of the solution of the substance being examined. Prepare a solution of the Standard Preparation in the same manner as described for the solution of the substance being examined and repeat the procedure beginning at the words ‘To a stoppered tube …’ but using 1.0 mL of this solution in place of the solution of the substance being examined.
Calculate the potency (PA) of the preparation being examined in Units per mg from the expression:
Where | A | = | volume in mL of 0.1m sodium thiosulfate VS used in the titration of the substance being examined, |
As | = | volume in mL of 0.1m sodium thiosulfate VS used in the titration of the Standard Preparation | |
B | = | volume in mL of 0.1m sodium thiosulfate VS used in the titration of the substance being examined inactivated by the addition of 1m hydrochloric acid, | |
Bs | = | volume in mL of 0.1m sodium thiosulfate VS used in the titration of the Standard Preparation inactivated by the addition of 1m hydrochloric acid, | |
Q | = | potency of the Standard Preparation in Units per mg | |
w | = | total weight in mg of the substance being examined in the solution prepared for assay, | |
ws | = | total weight in mg of the Standard Preparation in the solution prepared for assay. |