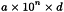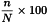Appendix XIV N3. Nucleated Cell Count and Viability
The determination of the quality of cell suspensions requires accurate measurements of both cell concentration and percentage of viable cells. These data are essential to the decision-making process for preparing cellular products and for maintaining optimum culture conditions. The cell count may be expressed as the number of cells per volume of cell suspension and the cell viability as the number of viable cells per volume of cell suspension. The cell-count procedure may be performed manually (haemocytometer) or with an automated apparatus (for example, particle counter, flow cytometer). Other methods than that described below may be used.
CELL NUMBER
manual counting
Description of the apparatus and test principle The following materials are required:
The haemocytometer is used to quantify the number of cells in a given solution by calculation of the cell concentration per millilitre (C) using the following expression:
| a | = | number of cells counted; |
| d | = | dilution factor (where applicable); |
| n | = | factor varying with the volume of the haemocytometer chamber. |
It is possible to distinguish between mixed cell populations provided they differ in size or pigmentation (for example, leukocytes and erythrocytes).
Preparation of the counting chamber and analysis Mount the coverslip (slightly moistened on the edges) on the slide. Move the coverslip back and forth over the slide, pressing slightly on the sides. Prepare a suitable dilution of the cell suspension in isotonic buffer or in haemolysis buffer.
Add an appropriate volume of the dilution to the counting chamber. The liquid is added to the border of the coverslip and is drained inside the chamber by capillarity. Carefully place the haemocytometer under the microscope and focus. Count the cells in a zone of the grid. Calculate the cell concentration in the diluted and original samples.
To increase the accuracy of the measurement, it is important to respect the following basic precautions:
automated counting methods
Particle counters based on conductivity variation Electronic particle counting devices measure the size and number of particles in a solution.
Particle counters are calibrated before use with a solution of particles of known concentration and size. To allow the counting of larger particles, tubes fitted with differently calibrated orifices are available. These apparatuses do not allow the discrimination between dead and live cells. As cell debris may also generate pulses that may cause errors, counters are also fitted with a threshold control allowing only larger particles to be counted.
The apparatus must be qualified for the counting of cellular products (in terms of linearity, accuracy, etc.).
Particle counters based on flow cytometry (2.7.24). The flow cytometer is calibrated with reference particles of known concentration and size to give an absolute cell number per volume. However, a calibrating solution is no longer necessary in instruments using 2 electrodes inserted in the sampling chamber where the fixed size of the sampling chamber and distance between the 2 electrodes allow the measurement of the content of a fixed volume. This type of instrument rarely needs to be calibrated after the initial setting.
VIABILITY
This section applies to cell staining by viability dyes and manual or automated analysis, under a light microscope or by flow cytometry, of a cell suspension in order to determine the percentage of viable cells.
Depending on the type of cells and the method used, the results may differ.
MANUAL DYE-EXCLUSION METHOD
Test principle This test is based on the exclusion of the dye from viable cells whereas dead or damaged cells absorb the dye and are coloured. It provides information on the cytoplasmic membrane integrity but its results do not necessarily reflect cell functionality. Recently trypsinised or thawed viable cells may have leaky membranes, causing them to absorb the dye.
Dye Trypan blue is the stain most commonly used to distinguish between viable and non-viable cells, but other suitable dyes such as erythrosin B or nigrosin may also be used. It is an acid dye (Mr 961), an anion with 4 sulfonate groups that can easily bind to proteins; therefore the protein concentration of the preparation to be tested must be as low as possible.
Test conditions Dye fixation is strongly influenced by pH, within a range of 6.6 to 7.6. Fixation is optimal at pH 7.5. The other conditions, such as the dye concentration and the staining time are validated.
Storage conditions of the dye Generally a 0.4 or 0.5 per cent trypan blue solution in sterile phosphate-buffered saline is used. Store protected from light and air.
Test preparation and analysis Stain the cell suspension at the required dilution (usually in phosphate-buffered saline) with, for example, a trypan blue solution having a final concentration of 0.1 to 0.2 per cent. Mix gently. Incubate for not more than 2-4 min at room temperature. Mix gently and place a suitable volume in a counting chamber. Count without delay.
Determine the percentage of viable cells from the ratio of the number of unstained cells to the total number of cells under a light microscope, considering all stained cells as dead cells. Viability (V) is calculated as a percentage using the following expression:
| n | = | number of unstained (viable) cells; |
| N | = | total number of cells (stained and unstained). |
It is essential that the incubation time be not more than 4 min as the number of stained cells may increase significantly afterwards. For a new determination, it may therefore be necessary to prepare a new test.
AUTOMATED METHODS
Flow cytometry
Test principle The test is based on the ability of certain dyes to cross damaged membranes and bind to DNA by intercalating between bases so that dead cells may fluoresce and be detected by flow cytometry (2.7.24). Non-viable cells are evaluated and discriminated by focusing on positive staining whereas viable cells remain unstained. This analysis is generally performed with 7-aminoactinomycin D (7-AAD) or propidium iodide (PI) but other suitable dyes may also be used.
Dye 7-AAD and PI are given as examples of membrane-impermeants that may be used as viability dyes.
7-AAD is an analogue of actinomycin D that contains a substituted amino group at position 7 of the chromophore. It intercalates between cytosine and guanine DNA bases. The spectral properties of 7-AAD make this molecule particularly suitable for flow-cytometry analysis. The maximum absorption of the 7-AAD/DNA complex is situated in the green spectral region and is thus suitable for an argon laser-equipped cytometer (excitation wavelength of 488 nm). The deep red fluorescence emission of the 7-AAD viability dye (635 nm to 675 nm) eases the use of the probe in combination with fluorescein isothiocyanate (FITC) and phycoerythrin (PE)-conjugated antibodies, because in contrast to PI, the 7-AAD/DNA complex shows minimal overlap with FITC and PE.
PI binds to double-stranded DNA by intercalating between bases with little or no sequence preference and with a stoichiometry of 1 dye molecule per 4-5 DNA base pairs. Once the dye is bound to nucleic acids, its fluorescence is enhanced 20- to 30-fold, the fluorescence excitation maximum is shifted around 30-40 nm towards the red and the fluorescence emission maximum (615 nm) is shifted around 15 nm towards the blue. Although its absorptivity is quite low, PI exhibits a sufficiently large Stokes shift to allow simultaneous detection of nucleic acids and fluorescein-labelled antibodies, provided that the suitable optical filters are used.
Storage conditions of nucleic acid dye solution 5 ± 3 °C.
Test preparation and analysis In the case of haematopoietic cells, the dye may be added after CD45 labelling to obtain a better separation of cells from debris and platelets with a side scatter (SS)/CD45+ gating region. The incubation conditions of the cell suspension with the dye are validated previously.
Incubation is performed at room temperature protected from light. Where necessary, lysis of red blood cells is performed using, for example, ammonium chloride. If not, add buffer alone.
Percentages of viable cells are directly given by the flow cytometer and deduced from the analysis of positive cells (dead cells) in the SS/7-AAD or SS/PI cytogram (dot plots).
Positive controls may consist of stabilised cells (dead cells) mixed with fresh viable cells at a target value.
Digital imaging of stained cells
Digital imaging allows the automation of dye-exclusion methods. The cell suspension and viability-dye solution are directly mixed by a machine. The system, which allows sample aspiration, reagent handling, and subsequent instrument cleaning is fully automated. Once the cellular suspension has been aspirated and mixed with the dye solution, it is pumped to the flow cell for imaging. The stained cell suspension is aspirated through a chamber where stroboscopic light allows a camera to photograph the flowing cells. The images are digitalised and the number of dead or live cells counted by the software.