Appendix XVI E. Microbiological Examination of Cell-based Preparations
1 INTRODUCTION
The approaches to microbiological examination of cell-based preparations outlined in this general chapter take into account the characteristics and limitations of these preparations, in particular their shelf-life, which may not always allow for completion of conventional microbiological examination tests before administration to the patient, as well as the amounts available for testing and sampling-related issues. These approaches may be applied when the test for sterility, described in general chapter 2.6.1. Sterility, is required but cannot be performed for technical reasons or due to the characteristics of the specific cell-based preparation.
1-1 SHELF-LIFE
The shelf-life of cell-based preparations is dependent on the cell characteristics and on the preservation conditions. For non-cryopreserved cell-based preparations, the shelf-life usually does not exceed 3-4 days and sometimes not more than a few hours. In such cases the microbiological status of the final preparation cannot be determined, according to general chapter 2.6.1, before the time of administration.
1-2 SAMPLE COMPOSITION
Microbial contaminants may be found either inside or on the surface of cells or other components of the cell-based preparation and may not be detected if only supernatants, such as culture or transport media, are analysed. The sample tested must be representative of all of the components of the cell-based preparation, unless otherwise justified.
1-3 SAMPLE SIZE
Due to constraints surrounding the use of a single donor or manufacturing-related capacities, the sample volume available for testing at the end of the production process may be limited. Nevertheless, with regard to the sampling error, which may lead to microbial contamination not being detected, the sample size must be sufficient to ensure suitable sensitivity and specificity of the chosen test method.
1-4 RATIONALE FOR METHOD SELECTION
Method selection must be based on the characteristics of the final preparation and the manufacturing process. To ensure safety for the intended use, the choice of method or combination of methods could be supported by a risk analysis of the potential exposure to microbiological contaminants, and the characteristics and intended use of the cell-based preparation. The media and incubation times used in these methods must be chosen taking into account the properties of the source material and the conditions during the manufacturing process that may support growth of specific micro-organisms (e.g. psychrophilic, thermophilic or fastidious bacteria or fungi). The composition of the cell-based preparation may impede certain test methods for physical reasons such as initial turbidity of the culture media after addition of the test sample.
The following approaches to microbiological examination may be applied:
2 GENERAL CONSIDERATIONS
2-1 GENERAL PRECAUTIONS
The test is carried out under aseptic conditions according to current regulations for potentially infective material. The precautions taken to avoid contamination are such that they do not affect any micro-organisms that are to be revealed in the test. The test is performed under working conditions that are monitored regularly by appropriate sampling of the working area and by carrying out appropriate controls. Testing shall take account of the potential for the presence of inhibitory substances in the sample that may affect the outcome of the test.
2-2 HANDLING CONSTRAINTS
2-2-1 Shelf-life
‘Negative-to-date’ is understood as an intermediate reading of a test method (2.6.1 or an automated growth-based method) that has not yet been completed. Where cell-based preparations have limiting shelf-lives, ‘negative-to-date’ results may be used as the readout, where justified. Based on the risk analysis of the characteristics and intended use of the cell-based preparation, results from additional microbiological in-process control may be needed at the time of use.
2-2-2 Sampling
The test sample must be representative of all of the components of the cell-based preparation and be taken from the final preparation. Where this is not possible, surrogate testing may be performed, for example on the liquids last in contact with the cells being processed.
3 METHODS FOR MICROBIOLOGICAL EXAMINATION OF CELL-BASED PREPARATIONS
3-1 AUTOMATED GROWTH-BASED METHOD
3-1-1 Growth promotion test
This section outlines the conditions for confirming the suitability of the culture media used for microbiological examination.
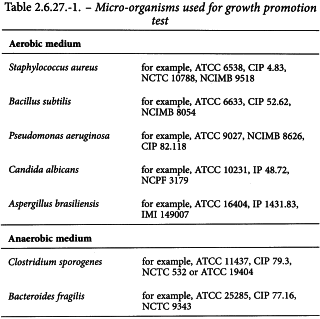
At least 2 suitable culture media intended for detection of fungi and aerobic and anaerobic bacteria are used. Each batch of sterile medium is tested for its growth-promoting capacities by inoculating duplicate test containers of each medium with not more than 100 CFU of each of the strains listed in Table 2.6.27.-1, and incubating for a maximum of 7 days for detection of microbial growth at the temperature defined for testing (see Table 2.6.27.-3). The test media are satisfactory if there is clear evidence of growth in all inoculated media containers within this incubation period.
3-1-2 Method suitability
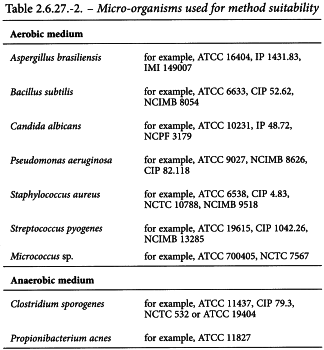
The test system is validated with respect to specificity (absence of false positive results), sensitivity, reproducibility and robustness. Regardless of the type of cell-based preparation, the manufacturing process, the sample volume analysed or the type of test system, the suitability of the method is to be confirmed in the presence of the specific sample composition. During the confirmation of the suitability of the method, particularly to determine sensitivity, the test is carried out using the micro-organisms listed in Table 2.6.27.-2. Sensitivity is meant as the capacity to detect 100 CFU or less. Not more than 100 CFU of the chosen micro-organisms are inoculated into the medium in the presence of the preparation to be tested, using at least 3 replicates. The microbial count in the micro-organism suspensions used for inoculation is determined by streaking an appropriate sample on agar plates. If between 1 and 100 CFU are detected for each strain within the duration of the assay, the method is valid for the intended sample composition.
It may be necessary to modify the list of micro-organisms in Table 2.6.27.-2 depending on the origin of the cells and any micro-organisms previously found or associated with the particular type of cells.
In some cases, the cell-based preparation itself can inactivate contaminating micro-organisms. Appropriate measures must be taken to ensure the suitability of any additional microbial strains used for method validation.
3-1-3 Testing of the preparation to be examined
Sample A sample that is representative of the characteristics of the cell-based preparation is tested. The sample is added to the culture medium as soon as possible. If it is necessary to store samples, the impact of the storage on potential contaminants is evaluated.
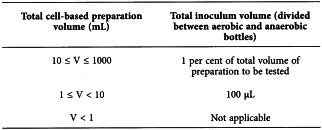
For cell-based preparations where the total volume (V) of the batch is between 1 mL and 1 L in a single container, the following table indicates the inoculation volume to be used.
For other volumes or multiple containers, alternative approaches should be used and have to be justified (see section 2-2-2). Enlargement of the total volume by means of dilution may be envisaged to assure complete inoculation of sample volumes of 100 µL. For preparation volumes less than 1 mL, where final sampling is not possible, surrogate testing, in-process testing or other appropriate testing should be used and has to be justified.
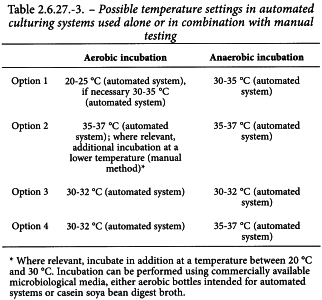
Analysis Samples are inoculated into containers of culture medium as soon as possible and incubated for not less than 7 days. Depending on the results obtained during method suitability testing and considering relevant micro-organisms, the incubation period may be extended up to 14 days. Selection of incubation temperatures should enable detection of a broad range of micro-organisms. This is typically in the range of 30-37 °C; however, for cell-based preparations with a very short shelf-life, a growth-accelerating temperature of not less than 35 °C may be more appropriate to obtain a relevant ‘negative-to-date′ readout of the test. In addition, for preparations where there is a significant risk of contamination from the environment, 2 temperature ranges of, for example, 20-25 °C (for aerobic) and 30-37 °C (for anaerobic) are used, in order to cover both environmental and clinical micro-organisms.
Table 2.6.27.-3 lists possible alternative approaches for the choice of incubation temperatures. The temperature and time for incubation are based on the results of the suitability study for the specific cell-based preparation.
3-1-4 Observation and interpretation of results
Media are examined, visually or with automated systems, at least daily and at the end of the observation period for evidence of microbial growth. If no growth is observed during or at the end of the observation period, the product is ‘culture negative′. If growth is observed in a valid test, the product is ‘culture positive′.
If the inoculated bottles are stored for more than 12 h before being placed into the automated culturing system, a subculture of each incubated bottle is performed to check for false negatives. This addresses cases in which, where a micro-organism is fast-growing and the conditions are optimal, micro-organisms may start to proliferate during storage. As a consequence, there may not be a significant increase in the relevant parameters at the time of testing and microbial contaminants may not be recognised by the system (false negative result).
3-2 ALTERNATIVE METHODS
3-2-1 Combination of preculturing and detection by alternative methods
The samples to be tested are incubated in both aerobic and anaerobic liquid cultivation media or equivalent solid media for a short period of time (e.g. 12-24 h depending on the sensitivity of the alternative approach used). An alternative method suitable for rapid detection of micro-organisms is then performed (e.g. nucleic acid amplification techniques (2.6.21), flow cytometry (2.7.24), bioluminescence (5.1.6)).
3-2-2 Direct detection by alternative methods (5.1.6)
Where a cell-based preparation has a very short shelf-life (e.g. a few hours) or where standard methods do not provide satisfactory detection of micro-organisms, non-growth-based, direct detection methods may be carried out for microbiological examination (e.g. nucleic acid amplification techniques (2.6.21), flow cytometry (2.7.24), bioluminescence (5.1.6)).
This approach enables a result to be obtained within a very short time, although at the expense of lower sensitivity, in comparison to growth-based methods. Depending on the approach used, both viable and non-viable micro-organisms may be detected.
3-2-3 Method validation
Validation is carried out according to the general recommendations of general chapter 5.1.6 and according to the recommendations specific to cell-based preparations in section 3-1-2 for automated growth-based methods. The sensitivity of these approaches must be validated considering the doubling times of potentially contaminating micro-organisms during pre-incubation.