SC IV L. Alternative Methods for Control of Microbiological Quality
The following chapter is published for information.
1 General introduction
The objective of this chapter is to facilitate the implementation and use of alternative microbiological methods where this can lead to efficient microbiological control and improved assurance for the quality of pharmaceutical products.
The microbiological methods described in the European Pharmacopoeia have been used for over a century and these methods for detecting, enumerating and identifying micro-organisms still serve microbiologists well. Over the years, these methods have been invaluable for the production of microbiologically safe pharmaceutical products. However, these microbiological methods are slow, and in the case of sterility tests, results are not available before an incubation period of 14 days. Consequently, the results from these methods seldom enable proactive corrective action to be taken.
Alternative methods for the control of microbiological quality have shown potential for real-time or near real-time results with the possibility of earlier corrective action. These new methods, if validated and adapted for routine use, can also offer significant improvements in the quality of testing.
Alternative methods may be used for in-process samples of pharmaceutical products, particularly for the application of Process Analytical Technology (PAT), for environmental monitoring and for industrial utilities (e.g. production and distribution of water, steam etc.), thereby contributing to the quality control of these products.
In this chapter, alternative microbiological methods for pharmaceutical application are described. For each method, the basic principle is stated and the advantages and disadvantages of the method are discussed along with any critical aspects to be considered. Potential uses that may be envisaged based on the principles of the method concerned are given, but it is not intended to suggest that such applications have been realised or that the list provided is exhaustive.
It is not the intention of this chapter to recommend one method over another, nor is it the intention to provide an exclusive or exhaustive list of alternative methods that can be used for pharmaceutical microbiological control. The information herein may be used, however, in the process of choosing an alternative microbiological method as a supplement or as an alternative to pharmacopoeial microbiological methods and to give guidance on validation of the chosen method. If a suitable method is described in the Pharmacopoeia, this method is the reference method. In this rapidly developing field, other methods are likely to appear and the guidance offered herein may be equally applicable in these cases.
There are 3 major types of determination specific to microbiological tests:
1-1 QUALITATIVE TESTS FOR THE PRESENCE OR ABSENCE OF MICRO-ORGANISMS
In conventional microbiological analysis, this type of test is characterised by the use of turbidity or other growth-related changes in a culture medium as evidence of the presence of viable micro-organisms in the test sample. The most common example of this test is the test for sterility (2.6.1). Other examples include those tests designed to evaluate the presence or absence of a particular type of viable micro-organism in a sample. The conventional sterility test may be replaced by, for example, tests based on bioluminescence or solid phase cytometry, gas detection or autofluorescence. Nucleic acid amplification techniques (NAT) (2.6.21) may also be used for the detection of mycoplasmas (2.6.7).
1-2 QUANTITATIVE TESTS FOR ENUMERATION OF MICRO-ORGANISMS
Membrane filtration and plate count methods are conventional methods used to estimate the number of viable micro-organisms present in a sample. The Most Probable Number (MPN) method is another example of such methods and was developed as a means of estimating the number of viable micro-organisms present in a sample not amenable to direct plating. Examples of alternative methods for enumeration include autofluorescence, flow cytometry, direct epifluorescent filter technique (DEFT) and solid phase cytometry.
1-3 IDENTIFICATION TESTS
Biochemical and morphological characterisation of an unknown micro-organism is the classical approach to identification. Recently developed methods have streamlined and automated aspects of this identification, especially in the areas of data handling, analysis and storage. Several alternative approaches that have been integrated into these methods include biochemical reactions, carbon substrate utilisation, characterisation of fatty acid composition, mass spectroscopy and Raman spectroscopy, restriction endonuclease banding patterns and the use of genome sequencing methods such as 16S rRNA gene sequence analysis for prokaryotes.
Traditional biochemical and phenotypic techniques have been shown to be less accurate and precise than genotypic methods.
Pure cultures are required for a precise identification and such cultures must be fresh and cultivated in appropriate media.
Databases are part of the systems and are included in the primary validation. As identification methods depend on the use of databases, the extent of coverage of the database with respect to the range of micro-organisms of interest must be taken into account during validation. Appropriate software allows customisation of the database, thereby allowing the user to add micro-organisms not previously included. This possibility must be considered during the validation.
2 GENERAL PRINCIPLES OF ALTERNATIVE METHODS
Alternative microbiological methods employ direct and indirect methods of detection; in some instances amplification of the signal is achieved by enrichment methods. In recognition of these differences, and for convenience within this chapter, alternative methods for the control of microbiological quality are divided into 3 categories:
In some instances, these distinctions are artificial, but enable a working classification to be created.
2-1 Growth-based methods
2-1-1 General critical aspects of methods based on early detection of growth
Such methods are critically dependent on microbial growth in order to provide an indication of the presence and/or number of micro-organisms. For the typically low levels of microbial contamination seen in pharmaceutical products, detection may take 24 h or longer. Increased sensitivity can be achieved with filtered products. In this case, after filtration, the membrane filter is incubated in or on the medium and the result is expressed as presence or absence in the quantity corresponding to the filtered volume. These systems, if they use an incubation step in liquid media, do not offer quantitative information, but a presence/absence determination in the quantity analysed. Analysis of more than one sample quantity may offer a semi-quantitative estimation (limit test). The major benefit of early detection methods compared to classical methods is often the capacity to simultaneously process a large number of samples and the potential to obtain a result in a shorter time.
The methods described below can be used for quantitative, semi-quantitative or qualitative analyses. They are also non-destructive, therefore subsequent identification of the micro-organism is possible.
2-1-2 Electrochemical methods
Principles of measurement Micro-organisms multiplying and metabolising in appropriate growth media produce highly charged ionic metabolites from weakly charged organic nutrients leading to the modification of electrical properties in such media. These changes in impedance (measured by conductance or capacitance) are monitored with electrodes included in the culture vessels and in contact with the culture medium. The measurable end-point is the time taken to detect a predetermined impedance change; for particular types of micro-organisms, the detection time is inversely proportional to the initial inoculum size. For yeasts and moulds, which only produce small changes in electrical impedance, an indirect measurement of conductance can be used. Direct measurement of capacitance can also be carried out.
Critical aspects There is no direct relationship between the original microbial level and the detectable end-point.
Potential uses Microbiological assay of antibiotics, efficacy of antimicrobial preservation and presence/absence testing.
2-1-3 Measurement of consumption or production of gas
Principles of measurement Appropriate growth media is utilised by actively multiplying and metabolising micro-organisms, leading to the production of metabolites or the elimination of specific nutrients. These methods detect microbial growth either by changes in the electrical properties of a sensor in response to a change in gas composition or by colorimetric changes of a sensor in response to physicochemical changes in the growth medium in contact with that sensor. The systems are based on non-destructive techniques which enable subsequent identification or strain typing of the micro-organisms. Bacteria and/or fungi may be grown in closed containers and continuous monitoring can be performed using automated instruments that measure gas evolution (e.g. CO2) or consumption (e.g. O2) as surrogate markers of microbial growth. Furthermore, the production of metabolites or elimination of nutrients can lead to changes in pH or redox potential. All of these changes can be measured either directly or indirectly as changes in colorimetric markers in the growth medium.
Critical aspects There is no direct relationship between the original microbial level and the detectable end-point. The incubation temperature, the physiological state and type of micro-organism, the initial load and the algorithm for data processing can significantly affect the results or the time to detection.
Potential uses Presence/absence testing of filterable or non-filterable samples (e.g. final drug products, in-process control samples, media fill or container closure integrity testing).
2-1-4 Bioluminescence
Principles of measurement Adenosine triphosphate (ATP) is a well-documented marker of cell viability. In this method, ATP first needs to be released from the micro-organisms using an appropriate extractant, followed by an assay using the luciferin/luciferase enzyme system, which emits light in proportion to the ATP present. The signal-to-noise ratio can be increased by addition of ADP and converting this ADP into released ATP.
Qualitative method: micro-organisms are cultivated in liquid medium. The emitted light is measured with a bioluminometer and is expressed in relative light units (RLU) (e.g. bioluminescence in a tube or a well of a microtitre plate). The RLU obtained from the sample is compared with a pre-determined threshold value. The result is positive if the RLU obtained with the analysed sample exceeds the threshold value.
Quantitative method: micro-organisms are captured on a membrane and cultivated by incubation on agar medium. Using a charge coupled device (CCD) camera, the ATP released from microcolonies can be detected by light emission and a quantitative determination is possible.
Critical aspects If the sample has a high level of bacterial contamination, the detection is rapid. For low levels of contamination, it is necessary to increase the number of micro-organisms using an incubation step in culture media (liquid or solid). The yield of ATP varies from one micro-organism to another and can depend on several factors including the species, the growth phase of the cell, the nutritional status, the cellular stress or the cellular age. Additional factors such as turbidity, sample colour or product matrix effects can also influence bioluminescence measurements. Extraction of ATP is generally a destructive process which should be considered with respect to any subsequent need for identification of detected micro-organisms.
Potential uses Presence/absence testing of filterable or non-filterable samples (e.g. final drug products, in-process control samples, media fill), total aerobic microbial count (TAMC), environmental and water monitoring, testing for efficacy of antimicrobial preservation.
2-1-5 Turbidimetry
Principles of measurement Microbial growth leads to detectable changes in medium opacity, which can be accurately quantified by optical density measurements at a specified wavelength. In its simplest form, such measurements are performed using a standard spectrophotometer, generally over a wavelength range of 420-615 nm. Alternative automated systems employ microtitre plate readers offering a continuous readout with early detection of optical density change.
Critical aspects Attempts have been made to extrapolate the initial microbial contamination from the time to detection, but this is limited to healthy micro-organisms with reproducible growth characteristics.
Potential uses By means of calibration graphs, determination of the inoculum size of microbial suspensions for use in pharmacopoeial tests. In automated mode, microbiological assay of antibiotics and testing for efficacy of antimicrobial preservation.
2-1-6 Growth detection using selective and/or indicative media
Principles of measurement The ability to detect the presence of specific enzymes using suitable chromogenic substrates has led to the development of a large number of methods for the identification of micro-organisms employing either manual or automated techniques. The incorporation of such substrates into a selective or non-selective primary isolation medium can eliminate the need for further subculture and biochemical testing for the identification of certain micro-organisms. Consequently, chromogenic liquid or solid culture media are designed to reveal specific enzymatic activities for detection and differentiation of micro-organisms. In these particular media, defined substrates are introduced into the formulation and are metabolised by the specific cell enzyme of a given bacterium or fungus during growth. These substrates, which are linked to coloured indicators, are chosen according to the diagnostic enzymatic activity sought. Furthermore, chromogenic broth can be used for early or improved detection of contamination (e.g. in media fill or broth-based detection methods).
The use of innovative media presents several advantages, namely improved discrimination of colonies in a mixed culture, ease of use and ease of interpretation. In addition, response times are shorter as the growth and identification of the micro-organism are simultaneous.
Critical aspects Validation of the media must be undertaken carefully to ensure a combination of specificity, selectivity and robustness. The quality of the signal is based not only on the careful choice of the enzymes or indicators used as the basis of detection (as these enzymes may be present in different micro-organism genera), but also on the physico-chemical characteristics of the medium, e.g. pH.
Potential uses Detection of specified micro-organisms and qualitative testing (e.g. media fill and container closure integrity testing) and quantitative testing (e.g. water testing).
2-2 Direct measurement
2-2-1 Solid phase cytometry
Principles of measurement Micro-organisms are stained for viability by exposure to a conjugated, initially non-fluorogenic, fluorophore. An intact cellular membrane is required to retain and accumulate the fluorophore within the cytoplasm. Inside metabolically-active microbial cells, the conjugate is enzymatically cleaved and the fluorescent derivative is released intracellularly. Micro-organisms are collected on a membrane filter either before or after viability staining.
Membrane surfaces retaining vital-stained cells are then scanned by a laser beam and epifluorescent excitation allows the detection of single, viable fluorescent micro-organisms. Appropriate software allows differentiation of viable micro-organisms from autofluorescent particles. The high sensitivity and rapidity of the method permit detection of microbial contaminants within a few hours. Total cell counts (viable and non-viable) can be obtained using fluorescent staining.
Critical aspects Metabolically active, fastidious and viable non-culturable micro-organisms can all be detected. This may result in reappraisal of the microbial limits established for the samples under evaluation. Spores require initiation of germination to enable detection. Single cell detection may be achievable, but identification of isolates might not be possible. False positives may occur due to autofluorescent particles that can be difficult to differentiate from micro-organisms. Signal discrimination and enhancement can be aided by microcolony growth.
Potential uses Rapid and sensitive method for the non-specific evaluation of microbial contamination.
2-2-2 Flow cytometry
Principles of measurement Fluorophore-labelled micro-organisms can be detected in suspension as they pass through a flow cytometer. Viable micro-organisms can be differentiated from non-viable particles by use of a viability-indicating fluorophore (see 2-2-1). The cell suspension stream is dispersed into a narrow channel and exposed to a laser which excites the fluorophore. Micro-organisms and particles are then counted in different channels depending on whether or not they contain a fluorescent cell.
Critical aspects Direct flow cytometry may be applied to the microbiological analysis of both filterable and non-filterable materials, and after possible enrichment in the case of the low contamination levels. It gives near real-time detection, but is not as sensitive as solid phase cytometry. To increase sensitivity for use in the pharmaceutical field, it is often necessary to add an incubation step in culture media, in which case the method becomes a combination of a growth-based method and a direct detection method. Particle size and number may have a significant effect on performance, and samples may require serial dilution. With the exception of filterability, similar considerations to those in solid phase cytometry apply. Clumping of bacteria can be a problem (e.g. Staphylococcus aureus).
Potential uses In contrast to solid phase cytometry, this method offers the potential to detect and enumerate microbial contamination in materials containing particulate matter and if the material cannot be filtered. If a pre-incubation step is needed, the method becomes a qualitative determination.
2-2-3 Direct epifluorescent filtration technique (DEFT)
Principles of measurement This technique may be considered a forerunner of solid phase cytometry. Micro-organisms, concentrated by filtration of the sample, are stained with a fluorescent dye (formerly acridine orange and now more commonly 4’,6-diamidino-2-phenylindole (DAPI)), that can be detected by epifluorescent illumination. Fluorescent vital staining techniques, as employed in solid phase cytometry (see 2-2-1), are amenable to DEFT, and fluorescent redox dyes such as 5-cyano-2,3-ditolyltetrazolium chloride (CTC) can be used to highlight respiring cells. Coupled with microscopy, the method allows rapid detection of micro-organisms with an absolute sensitivity that is dependent on the volume of product filtered and the number of fields of view examined. Semi-automated auto-focusing systems coupled to image analysis have served to improve the utility of this method. A modification of the principle involves sampling using an adhesive sheet (which permits collection of cells from surfaces), subsequent staining on the sheet itself, followed by direct observation using an epifluorescence microscope.
Critical aspects The distribution of micro-organisms on the membrane affects method robustness. The intensity of fluorescence can be influenced by the staining process and the metabolic status of the micro-organisms. Fluorescence is not necessarily an indicator of viability. A brief period of culture on the filter surface prior to staining allows microcolony formation; these microcolonies stain readily, can be easily enumerated and are demonstrable evidence of viability.
Potential uses DEFT is generally limited to low viscosity fluids, although pre-dilution or pre-filtration has occasionally been applied to viscous or particulate products. Monitoring of microbial contamination has been successfully applied to aqueous pharmaceuticals.
2-2-4 Autofluorescence
Principles of measurement The presence of endogenous autofluorescent molecules and metabolites (e.g. NADPH, flavoproteins) within micro-organisms allows the early detection and quantitative enumeration of microcolonies or single cells. For direct measurements, the laser-induced autofluorescence of a single micro-organism is captured by a detector, while for growth-based systems, automated sequential imaging of the membrane surface on agar medium over the incubation period is employed and image overlay allows differentiation of growing microcolonies from fluorescent particulates. The emitted light is detected by a CCD camera. Non-destructive detection allows identification of contaminants at the end of the incubation period.
Critical aspects For a non-growth based measurement, viable, but non-culturable, micro-organisms might be detected. It may be difficult to distinguish between culturable micro-organisms, viable but non-culturable micro-organisms and/or other particles.
Potential uses Environmental monitoring, filterable in-process samples, water testing and product release for both sterile and non-sterile applications.
2-3 CELL COMPONENT ANALYSIS
2-3-1 Phenotypic techniques
2-3-1-1Immunological methods
Principles of measurement Antibody-antigen reactions can be employed to detect unique cellular determinants of specific micro-organisms. These reactions can be linked to agglutination phenomena and colorimetric or fluorimetric end-points, which offer both quantitative and qualitative detection. Enzyme-linked immunosorbent assays (ELISA) offer simple solid-phase methodologies.
Critical aspects Immunological detection methods depend on the unique expression of specific identifiers, but do not necessarily demonstrate the presence of viable micro-organisms.
Potential uses Detection and identification of specified micro-organisms.
2-3-1-2Fatty acid profiles
Principles of measurement The fatty acid composition of micro-organisms is stable, well conserved and shows a high degree of homogeneity within different taxonomic groups. The isolate is grown on a standard medium and harvested. The fatty acids are saponified, methylated and extracted, and the occurrence and amount of the resulting fatty acid methyl esters are measured using high-resolution gas chromatography. The fatty acid composition of an unknown isolate is compared with a database of known isolates for a possible match and identification.
Critical aspects The use of fatty acid profiles for microbial identification requires a high degree of standardisation. It is critical for the fatty acid composition of microbial cells that isolates are grown using standardised media and standard incubation conditions. Standard conditions for operation of the gas chromatograph must also be employed, with frequent runs of calibration standards and known isolates being very important.
Potential uses Identification or characterisation of environmental and product microbial contamination (for contaminant tracing and detection of specified micro-organisms).
2-3-1-3Fourier transform infrared (FTIR) spectroscopy
Principles of measurement A Fourier transformation of the infrared spectrum of whole micro-organisms gives a stable, recognisable pattern typical of the taxonomic groups of micro-organisms. The analysis of the FTIR pattern can be performed with commercially available instruments. The isolate is grown on a standard medium and harvested. Cell mass is transferred to a carrier, and the infrared spectrum is recorded. The Fourier transformation is calculated and the pattern is compared with a database of known isolates for a possible match and identification.
Critical aspects The use of FTIR patterns for microbial identification requires a high degree of standardisation. It is critical for the FTIR pattern of microbial cells that isolates are grown using standardised media and standard incubation conditions. The cells must be in the same state of the growth cycle when analysed, and particular attention must be paid to this in the validation process.
Potential uses Identification or characterisation of environmental and product microbial contamination (for contaminant tracing and detection of specified micro-organisms).
2-3-1-4Mass spectrometry
Principles of measurement Ionised particles released by exposing microbial isolates to a laser in a vacuum can be analysed by mass spectrometry, providing characteristic spectra. Similarly, intact microbial cells, when subject to intense ionisation under matrix-assisted laser desorption ionisation-time of flight (MALDI-TOF) mass spectrometry, release a distinctive pattern of charged species. Such spectra can be compared with known profiles.
Critical aspects The isolates must be cultured under standardised conditions prior to analysis.
Potential uses Identification or characterisation of environmental and product microbial contaminants (for contaminant tracing and detection of specified micro-organisms).
2-3-1-5Biochemical assays based on physiological reactions
Principles of measurement Systems capable of performing biochemical assays based on physiological reactions are used for the identification of micro-organisms. In the presence of a pure colony, the five basic steps for these assays are preparation, inoculation, incubation, readings and interpretation. These steps are usually preceded by a description of the colony morphology, a differentiation test (e.g. Gram stain), a description of the cellular morphology and/or other early biochemical differentiation tests (e.g. oxidase, catalase, coagulase) in order to determine the appropriate testing protocol.
The Gram stain is often a key characteristic upon which further testing is based. Alternatives to the traditional staining method include the potassium hydroxide (KOH) string test, the aminopeptidase test, a fluorescent staining method and a limulus amoebocyte lysate (LAL) based assay. Test kits are available for the latter 3 methods. The fluorescent staining method requires a fluorescence microscope or a flow cytometer.
Microbial cell suspensions are tested using biochemical (assimilation or susceptibility) test kits (plates or strips). Anaerobic and aerobic micro-organisms develop characteristic reactions to selected biochemical substances. They are also known to utilise specific carbon, nitrogen, phosphorus and sulfur sources or to be inhibited by a specific concentration of an antimicrobial agent. The results are based on measurable changes (e.g. turbidity, chromogenic or fluorogenic reaction) due to the growth or inhibition of the micro-organism under investigation. Comparison of the metabolic and/or antimicrobial resistance profile with a database allows for identification of the culture. These methods can be performed manually or by semi- or fully automated instruments. Complementary tests can be performed in cases of poor discrimination. Subcultures can help in cases of indeterminate results.
Critical aspects A fresh physiological culture is required. The performance of the system is also dependent on the selected phenotypic parameters, which must be stable, significant and in sufficient number.
Potential uses Identification or characterisation of environmental and product microbial contamination (for contaminant tracing and detection of specified micro-organisms).
2-3-2 Genotypic techniques
Identification and detection of micro-organisms as well as characterisation of strains belonging to the same species may be achieved by direct detection of nucleotide target sequences that are unique for a particular microbial species or microbial group, and are targets of the genotypic (DNA or RNA-based) detection techniques. These detection techniques may be separated into 3 broad categories: direct hybridisation, nucleic acid amplification and genetic fingerprinting.
2-3-2-1Direct hybridisation
General principles of measurement DNA probes are short, labelled, single-strand segments of DNA that hybridise with a complementary region of microbial DNA or RNA. The probe or target DNA is usually labelled with either radioactive, fluorescent or chromogenic molecules in order to provide a hybridisation signal. Hybridisation assays include fluorescence in situ hybridisation (FISH) and microarray-based techniques.
General critical aspects Hybridisation generally requires a large amount of the target DNA for analysis, which may result in lower detection sensitivity. The availability of suitable probes may be limited.
Potential uses Due to the high specificity of the sequence-based hybridisation reaction, this method may be used for both detection and identification of micro-organisms.
2-3-2-2Nucleic acid amplification techniques (NAT)
General principles of measurement NAT relies on the reiteration of the DNA polymerisation process, leading to an exponential increase of a specific nucleic acid fragment. The polymerase chain reaction (PCR) is the most widely used method for target DNA amplification. In this cyclic process, a specific DNA fragment is copied by a thermostable DNA polymerase enzyme in the presence of nucleotides and oligonucleotide primers, previously designed to flank the target sequence and to hybridise with it (see also general chapter 2.6.21). After PCR, the amplified nucleic acid targets can be analysed using several methods of post-amplification analysis: fragment size analysis in gel electrophoresis, DNA sequencing or specific detection by hybridisation with a fluorescent-labelled probe. Real-time PCR eliminates the need for further post-amplification processing and offers the additional advantage that the likelihood of cross-contamination is minimised. An important advantage of real-time PCR is the ability to quantify the starting amount of the DNA target sequence in the original sample, in contrast to conventional PCR techniques, which are based on end-point detection. Since the amount of PCR product detected at the beginning of the exponential phase of the amplification reaction correlates with the initial starting amount of the DNA target, modern real-time PCR techniques have been developed to measure this exponential phase of the reaction. Automated real-time PCR systems are commercially available. For identification of species, either species-specific probes or primers can be used.
RNA can also be amplified by both conventional and real-time PCR after transcription into cDNA using a reverse transcriptase enzyme. This technique is known as reverse transcriptase PCR (RT-PCR) and it enables detection and identification of RNA viruses or viable organisms. Alternatively, specific RNA-based amplification techniques, for example nucleic acid sequence-based amplification or transcription-mediated amplification, are available. Both techniques produce RNA amplicons, in contrast to PCR which only produces DNA amplicons, even when starting from an RNA target.
Types of target to be amplified Regardless of the type of NAT used, the specificity of the test is determined by the target DNA sequence under evaluation. For identification/characterisation purposes, the 16S or 23S ribosomal RNA genes may be used as targets. The 16S rRNA gene is an evolutionary-conserved gene present in all bacterial species, and is a broad range target as it is a universal marker for bacterial detection. The 23S rRNA gene is not widely used as a single target, but the 16S-23S rRNA transcribed intergenic spacer regions can be employed to distinguish between certain closely related species and/or to identify subtypes. Alternative broad-range targets include the groEL and tuf genes. Apart from broad-range targets, species-specific sequences can be used as targets for micro-organism identification. Depending on the species, either specific surface antigens, virulence factors or genes which code toxins may be amplified to detect and identify micro-organisms.
Depending on the aim, a choice must be made between amplification of either a DNA or an RNA target, as this target choice affects the correlation with viability. DNA targets are generally more widely used for identification purposes, but the use of DNA as a marker has the disadvantage that dead micro-organisms can also be detected. As mRNA is rapidly degraded in dead cells, it is considered a marker for viability. Furthermore, mRNA is the obligatory target for the identification of RNA viruses.
Critical aspects of (semi-) quantitative detection by real-time PCR Quantification of the target requires generation of appropriate standards and the use of standardised procedures.
Critical aspects of RT-PCR RNA is less stable compared to DNA, so it requires more attention during processing. Depending on the quality of the RNA isolation, the efficiency of the cDNA synthesis can vary. RT-PCR can be used to specifically detect RNA if DNA contamination of the RNA sample is low.
Critical aspects of using the 16S or 23S rRNA gene as a target for species identification 16S rRNA gene sequencing is a valuable method for identification of bacteria provided that appropriate universal primers from databases are selected. Its discriminatory power depends on the variability and the length of the 16S rRNA gene within a certain species. Regarding the use of assays targeting the 16S-23S rRNA intergenic spacer regions, the choice of appropriate species-specific primers/probes is of critical importance due to the potential polymorphism of such regions.
Potential uses of NAT Due to the high sensitivity and specificity of amplification techniques, they are suitable for both detection and identification of micro-organisms. Real-time PCR is needed for quantitative or semi-quantitative analysis of the target. Besides quantitative determinations, the real-time PCR technique allows simultaneous detection of multiple targets in a single sample, as long as appropriate primers and probes that allow for multiplexing are employed. The sequencing of different genes (e.g.16S rDNA, 23S rDNA, rpoB, Gyr) is best applied to the identification of micro-organisms.
2-3-2-3Genetic fingerprinting
Principles of measurement Genetic fingerprinting is the identification of a strain on the basis of its DNA profile (or RNA for RNA viruses). Individual DNA profiles may be different due to genetic diversity between strains of the same species, and the aim of the fingerprinting methods is to discriminate between these strains. The classical genetic fingerprinting technique characterises micro-organisms using restriction fragments of chromosomal DNA from bacterial and fungal genomes.
Different strains from the same species may exhibit different patterns and these differences are referred to as restriction fragment length polymorphisms (RFLPs). As cutting the chromosomal DNA with restriction enzymes generates too many fragment bands to be efficiently and accurately compared, several modifications of the conventional RFLP-based method have been developed. Examples of the kind of technologies used are ribotyping, pulsed-field gel electrophoresis (PFGE) and amplified fragment length polymorphism (AFLP). Several other fingerprinting methods use PCR to selectively amplify defined subsets of DNA restriction fragments from the entire genome, for example random amplified polymorphic DNA (RAPD) and variable number tandem repeats (VNTR).
Critical aspects All fingerprinting techniques require that the micro-organism is present as a pure culture. Depending on the method, a preliminary enrichment cultivation step may be necessary if a defined quantity or a specific DNA preparation is required for the test, e.g. AFLP and PFGE. The discriminatory power, the reproducibility, the expertise needed and the labour-burden vary among techniques. The major criticism of conventional RFLP analysis is the complexity of the banding patterns. The discriminatory power of ribotyping (based on patterns of rRNA genes) is less than that of PFGE (based on patterns of the whole genomic DNA) or some PCR-based methods, but it has the advantage that it can be a highly automated system. Although PFGE is one of the most highly discriminatory fingerprinting methods, it is time-consuming and technically demanding in the laboratory as it is not automated. It also requires the use of standardised protocols. AFLP has high reproducibility, but requires technical expertise and the interpretation of results may need automated computer analysis. The reproducibility of RAPD may be poor, so it must be performed in a standardised way.
Potential uses Genetic fingerprinting methods are mainly used for strain discrimination (characterisation below species level). They are a powerful tool for investigating and tracing the source and the spread of microbial contamination.
3 VALIDATION OF ALTERNATIVE MICROBIOLOGICAL METHODS
3-1 INTRODUCTION
Validation, whilst subject to a variety of context-specific definitions, can be generally defined as a method to establish documented evidence that a process will consistently achieve its intended goal. Therefore, to validate an alternative microbiological method, it is essential to understand and define what the procedure is intended to achieve.
Typically, pharmaceutical microbiological methods use specific characteristics of micro-organisms as indicators or detection principles in order to determine microbiological quality. The information generally sought is presence/absence, number, viability and/or identity of micro-organisms in a given product or environment. Any given method will usually provide an indirect and conditional measure of microbiological quality. For example, the total number and viability of micro-organisms can be indicated by the number of colonies appearing under a certain set of conditions of sample preparation, cultivation and incubation; reproduction in classical microbiology is hence taken as the general indicator for viability. There are other parameters, however, that can be used as a viability measure, such as the level of ATP or the accumulation or metabolism of substrates in living cells. The results from different viability-indicating methods may not always be identical; micro-organisms may not be able to reproduce on a given medium, but may still accumulate and metabolise a substrate. Conversely, micro-organisms may be unable, at a given state of damage, to accumulate a substrate, but may still be able to recover and reproduce.
Similar considerations arise with the multiplicity of methods used for identification of micro-organisms. Therefore, while characterisation of the pattern of metabolic activity is frequently used for species identification, alternative methods also exist. Again, the outcomes obtained may not be fully consistent for the different identification methods, as one answer may be appropriate for the construction of a correct phylogenetic correlation tree, while another may be more useful in the context of pathogenicity or other property of the differentiated micro-organisms.
3-2 VALIDATION PROCESS
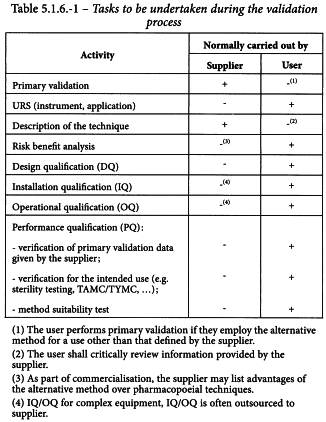
Two levels of validation must be envisaged for the application of alternative microbiological methods, namely primary validation and validation for the intended use. The supplier of the alternative technology typically performs primary validation of a method, whereas validation for the intended use, which is a verification of the suitability or applicability of the method in a given situation, must be seen as the responsibility of the user.
Where specific equipment is critical for the application of a method, the equipment, including computer hardware and software, must be fully qualified.
3-2-1 Description of the technique
In order to characterise a specific microbiological method, the principle of detection must be clearly described by the supplier. Through primary validation, the method must be fully detailed with respect to the conditions required for application, the materials and equipment needed and the expected signal. The user shall critically review the available information.
3-2-2 Risk-benefit analysis
For validation of specific alternative microbiological methods, it is critical that the purpose of the quality assurance procedure is precisely outlined, as this defines the type and depth of information needed. The information obtained by, and the limitations of, the pharmacopoeial method and the alternative method must be considered and compared in a risk-benefit analysis.
The risk level in adopting an alternative method varies depending on the technology considered, the methodology it replaces, the nature of the measurements taken (qualitative, quantitative or identification), the particular product or process attribute being evaluated, the location of the measurement in the manufacturing process chain and various other factors.
Risk analysis tools may be utilised in order to determine which alternative method is to be implemented, to assist in the justification of its implementation or to better understand the impact of implementation on production and/or product quality. An alternative method can be justified for use if the information obtained gives a scientifically sound measure of microbiological quality, and if the limitations of the method are not more severe than those of the pharmacopoeial method.
3-2-3 Primary validation
The supplier, using a panel of test micro-organisms appropriate for the intended use, must characterise the principle of detection. Depending on the type of alternative method, relevant validation criteria shall be selected from those listed below:
3-2-4 Validation for the intended use
Validation for the intended use should encompass the entire process, from the decision to change any aspects of a microbiological testing programme to on-going routine use. It should consist of the following phases:
The supplier and user have different tasks to perform with regard to the validation and implementation of an alternative method. These tasks are summarised in Table 5.1.6.-1.
3-2-4-1User requirement specification (URS)
The URS describes the functions that the method must be capable of performing and will form the basis of the method selection process. It is an essential document, as acceptance testing will be based on the requirements detailed therein. It is important to consider data management capabilities at this stage, particularly within a regulatory context. The URS shall at least address the following items:
3-2-4-2Design qualification (DQ)
The DQ provides documented evidence that the design of any associated equipment is suitable for correct performance of the method. Most alternative method systems are based on commercial off-the-shelf equipment. The DQ is most suitably performed, therefore, by the instrument developer/manufacturer. Nevertheless, the user shall verify that the equipment meets the specifications laid down in the URS for the intended application.
3-2-4-3Installation qualification (IQ)
The IQ provides documented evidence that the equipment has been provided and installed in accordance with its specifications.
3-2-4-4Operational qualification (OQ)
The OQ provides documented evidence that the installed equipment operates within predetermined limits when used in accordance with its operational procedures.
3-2-4-5Performance qualification (PQ)
The PQ provides documented evidence that the method, with the equipment installed and operated according to operational procedures, consistently performs in accordance with predetermined criteria and thereby yields correct results for the method. This is typically done with a panel of micro-organisms (e.g. pharmacopoeial test strains, in-house isolates or stressed/slow-growing micro-organisms). This assures that the conditions employed by the user laboratory make it possible to satisfy the criteria described by the supplier of the method in the model system used for the primary validation.
Verification of primary validation data given by the supplier (see 3-2-3). The method is verified using the panel of test micro-organisms given by the corresponding pharmacopoeial chapter. The alternative method must be applied according to the specified procedure of the supplier, without the samples to be analysed under the responsibility of the user, and must be shown to give comparable results as characterised in the model system used by the supplier.
Verification for the intended use (e.g. sterility testing, total aerobic microbial count (TAMC)/total combined yeasts/moulds count (TYMC), etc) The following points, where applicable, should be addressed:
Acceptance criteria for the method will need to be defined as a function of the application and the validation data.
3-3 TYPES OF MICROBIOLOGICAL TESTS
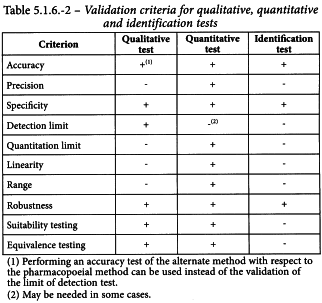
Validation of a microbiological method is the process whereby it is experimentally established by the user that the performance characteristics of the method meet the requirements of the intended application. As microbiological tests have 3 basic applications (qualitative, quantitative and identification), 3 separate sets of validation criteria are required. These criteria are described below and summarised in Table 5.1.6.-2.
3-3-1 Validation of alternative qualitative tests for the presence or absence of micro-organisms
3-3-1-1Specificity
The specificity of an alternative qualitative method is its ability to detect only the required micro-organisms, i.e. does not generate false positive results. This can be demonstrated using a panel of appropriate micro-organisms. Where relevant for the purpose of the test, mixtures of micro-organisms are used during validation. For qualitative methods that rely on growth to demonstrate presence or absence of micro-organisms, specificity is adequately addressed by demonstrating the growth promotion properties of the media. For those methods that do not require growth as an indicator of microbial presence, the specificity assures that extraneous matter in the test system does not interfere with the test.
3-3-1-2Detection Limit
The detection limit of an alternative qualitative method is the lowest number of micro-organisms in a sample that can be detected under the stated analytical conditions. A microbiological limit test determines the presence or absence of micro-organisms in a defined quantity of the sample under test. Due to the nature of microbiological tests, the detection limit reflects the number of micro-organisms present in the original sample before any dilution or incubation steps. The detection limit of the alternative method must not be a number greater than that of the pharmacopoeial method.
It is essential that the detection limit is determined using a sufficient number of replicates and a number of independent determinations.
3-3-1-3Robustness
The robustness of an alternative qualitative method is a measure of its capacity to remain unaffected by small but deliberate variations in method parameters (e.g. incubation period or incubation temperature range). Robustness is a validation parameter best suited to determination by the supplier of the method. Nevertheless, if the user modifies critical parameters, any effect on robustness must be evaluated. Robustness of a qualitative method is judged by its ability to detect the test micro-organisms after deliberate variations to the method parameters.
3-3-1-4Suitability testing
The alternative method must be applied according to the specified procedure and with the samples to be analysed under the responsibility of the user. It must be shown that the test sample does not interfere with the system′s detection capacity or microbial recovery. Specific points to be addressed are:
Acceptance criteria for the method in routine use will need to be defined as a function of the application and the validation data.
3-3-1-5Equivalence testing
Equivalence testing of 2 qualitative methods can be conducted directly on the validation parameters. This approach requires an adequate comparison experiment at low levels of inoculation (e.g. less than 5 CFU) with sufficient numbers of replicates for relevant strains of test micro-organisms. Alternatively, and in some cases additionally, equivalence testing can be carried out by the parallel testing of a predefined number of samples or for a predefined period of time. This parallel testing can be justified based on a risk assessment. The alternative method must enable an unequivocal decision as to whether compliance with the standards of the monographs would be achieved if the official method was used.
3-3-2 Validation of alternative quantitative tests for enumeration of micro-organisms
3-3-2-1Accuracy
The accuracy of an alternative quantitative method is the closeness of the test results obtained by the alternative method to those obtained by the pharmacopoeial method. Accuracy must be demonstrated across the practical range of the test. It is usually expressed as the percentage recovery of micro-organisms by the alternative method compared to the percentage recovery using the pharmacopoeial method, taking into account statistical analysis.
Accuracy may be shown by preparing and testing a suspension of micro-organisms at the upper end of the test range and serially diluting to the lower end of the test range. For example, if the alternative method is meant to replace the pharmacopoeial plate count method for viable counts, then a reasonable range might be 100-106 CFU/mL. If instead, it is a replacement for the MPN method, a much narrower range may be used. At least 1 suspension for each test micro-organism dilution must be analysed.
The alternative method should be shown to recover at least as many micro-organisms as the pharmacopoeial method using appropriate statistical analysis.
The protocol used to check the linearity of the method (see 3-3-2-5) may also be used to check the accuracy. The suspensions of micro-organisms prepared for the alternative method are counted at the same time using the pharmacopoeial method.
3-3-2-2Precision
The precision of an alternative quantitative method is the degree of agreement between individual test results when the procedure is applied repeatedly to multiple samplings of homogeneous suspensions of micro-organisms under the prescribed conditions. Precision should be split into repeatability and intermediate precision under normal or routine operating conditions. Repeatability (also referred to as within-run variability) refers to the use of the microbiological method with the same sample (replicate) in the same laboratory over a short period of time with the same analyst and the same equipment. It gives the minimum variability of the method. Intermediate precision (includes run-to-run variability and within-run variability) refers to the use of the microbiological method applied to different sample preparations of the product under test in the same laboratory with different analysts, equipment and/or on different days. It gives the maximum variability of the method. The precision of a microbiological method is usually expressed as the standard deviation or relative standard deviation (coefficient of variation). At least 1 suspension in the middle of the test range is analysed. The number of replicates is chosen so that the entire test can be carried out during the same working session, i.e. under the same operating conditions and without any change in the suspension of micro-organisms. For intermediate precision, other working sessions are then carried out under conditions of maximum variability (different reagents, operators and/or days, etc.). The variance in the results observed in each of the working sessions is calculated. If the variances are homogeneous, the variance of the repeatability can be calculated. The inter-group variance of the results is also calculated and the resultant variance of the intermediate precision is given as the sum of the variance of the repeatability and the inter-group variance. The coefficient of variation is then calculated. Alternative methods must demonstrate precision comparable to that of the pharmacopoeial methods.
3-3-2-3Specificity
The specificity of an alternative quantitative method is its ability to quantify only the required micro-organisms, i.e. does not generate false positive results. This may be demonstrated using a panel of appropriate micro-organisms. Where relevant for the purpose of the test, mixtures of micro-organisms are used during validation. For those methods that do not require growth as an indicator of microbial presence, the specificity assures that extraneous matter in the test system does not interfere with the test.
3-3-2-4Quantitation limit
The quantitation limit of an alternative quantitative method is the lowest number of CFUs in a sample which can be quantitatively determined with suitable precision and accuracy. It is essential that the quantitation limit is determined from a number of replicates. The results of the linearity and accuracy studies can also be used. In this case, the lowest concentration in the linear range is considered to be the quantitation limit of the method. The quantitation limit of the alternative method must not be greater than that of the pharmacopoeial method.
3-3-2-5Linearity
The linearity of an alternative quantitative method is its ability (within a given range) to produce results that are proportional to the concentration of micro-organisms present in the sample. The linearity must be determined over a reasonable range (e.g. 100-106 CFU/mL) so as to correspond to the purpose of the alternative method. One approach would be to select different concentrations of each test micro-organism and test several replicates. For each concentration, an appropriate number of replicates is chosen to confirm linearity. The number of replicates is chosen so that the entire test can be carried out during the same working session. After checking the homogeneity of the variances of the results obtained for each concentration, the regression line is calculated. Linearity is demonstrated if the estimated slope is significant and if the test for deviation from linearity is non-significant (see general chapter 5.3).
3-3-2-6Range
The range of an alternative quantitative method is the interval between the upper and lower levels of micro-organisms as determined from the related studies of precision, accuracy and linearity using the specified method; it is dependent on the intended application.
3-3-2-7Robustness
The robustness of an alternative quantitative method is a measure of its capacity to remain unaffected by small but deliberate variations in method parameters (e.g. incubation period or incubation temperature range). Robustness is a validation parameter best suited to determination by the supplier of the method. Nevertheless, if the user modifies critical parameters, the effects on robustness must be evaluated. Robustness of an alternative quantitative method is judged by its ability to accurately enumerate the test micro-organisms after deliberate variations to the method parameters.
3-3-2-8Suitability testing
The alternative method must be applied according to the specified procedure and with the samples to be analysed under the responsibility of the user. It must be shown that the test sample does not interfere with the system′s enumeration capacity or microbial recovery. Specific points to be addressed are:
Acceptance criteria for the method are defined as a function of the application and of the validation data.
3-3-2-9Equivalence testing
Equivalence testing of 2 quantitative methods can be conducted directly on the validation parameters. This approach requires an adequate comparison experiment at low levels of inoculation (e.g. less than 5 CFU) with sufficient numbers of replicates for relevant strains of test micro-organisms. Alternatively, and in some cases additionally, equivalence testing can be carried out by the parallel testing of a predefined number of samples or for a predefined period of time. This parallel testing can be justified based on a risk assessment.
If the result of the alternative method can be expressed as the number of CFUs per weight or per volume, statistical analysis of the results shall demonstrate that the results of the alternative method enable an unequivocal decision as to whether compliance with the standards of the monographs would be achieved if the official method was used.
If the result of the alternative method cannot be expressed as the number of CFUs, equivalence testing is performed using suitable parameters, followed by statistical analysis to demonstrate that the results of the alternative method enable an unequivocal decision as to whether compliance with the standards of the monographs would be achieved if the official method was used.
3-3-3 Validation of alternative identification tests
There is a large body of evidence that different methods vary considerably in their ability to identify micro-organisms. It must be accepted that a method of identification needs to be internally consistent, but may differ from others in its identification of micro-organisms.
3-3-3-1Accuracy
The accuracy of an alternative identification method is its ability to identify the desired micro-organism to the required taxonomic level. It must be demonstrated using well-characterised reference micro-organisms, e.g. type strains. Accuracy of the identification method is usually expressed as the number of correct identifications divided by the total number of identifications.
3-3-3-2Specificity
The specificity of an alternative identification method is its ability to discriminate micro-organisms actually present from interfering factors that cause false identification results. Such factors include chemical substances and mixtures of micro-organisms, which cause the test to identify micro-organisms not actually present in the sample material (e.g. the presence of mixtures of DNA material from 2 micro-organisms in a sequencing test leading to the false identification of a third micro-organism).
3-3-3-3Robustness
The robustness of an alternative identification method is a measure of its capacity to remain unaffected by small but deliberate variations in method parameters (e.g. incubation period or incubation temperature range). Robustness is a validation parameter best suited to determination by the supplier of the method. Nevertheless, if the user modifies critical parameters, the effects on robustness have to be evaluated. Robustness of an identification method is judged by its ability to correctly identify the test micro-organisms after deliberate variations to the method parameters.