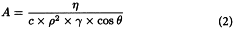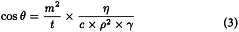Appendix XVII T. Wettability of Porous Solids Including Powders
INTRODUCTION
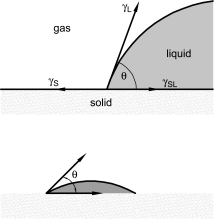
The wettability of solid surfaces is commonly characterised by direct or indirect contact angle measurements. The contact angle (θ) between a liquid and a solid is the angle naturally formed when a drop of a liquid is placed on a solid surface. This is depicted in Figure 2.9.45.-1. For a given liquid, wettable solids show a low contact angle and non-wettable solids show a contact angle of 90° or more.
2 methods for the determination of wettability are described below. The methods are capable of measuring the wettability of porous solids like powders or granules. Both methods express the wettability by a contact angle measurement between the porous solid and a given liquid.
The sessile drop method is based on direct measurement of a contact angle of a sessile drop on a compacted powder disc.
With the Washburn method the contact angle is indirectly measured. The method is based on the capillary effect of the powder pores. The effect (mass gain) is recorded by special electronic balances starting the moment when the powder sample touches the surface of a liquid, preferably not dissolving or poorly dissolving the sample. The measurement has very little or no effect on the state of the powder.
Any pre-treatment of the sample to be examined is disadvantageous, since the properties may be significantly altered. For example, the compaction of a powder as a disc may decrease the surface free energy when the crystalline state of the powder is changed (e.g. metastable forms), or may increase surface free energy by creating crystal defects (disadvantage of the sessile drop method since compacted powder discs are tested).
The methods are usually applied to examine the following parameters:
SESSILE DROP METHOD
This method may be used to characterise directly the wettability of coatings and compacted formulations such as tablets. Moreover, it is sometimes possible to use the sessile drop instrument in a dynamic measurement (dynamic contact angle measurement, Figure 2.9.45.-2) of porous solid/liquid systems where the contact angle decreases. By taking several contact angle measurements as a function of time, the rate of spreading accompanied by penetration of a liquid droplet into a slightly porous solid may be studied.
Under equilibrium conditions the contact angle of a sessile drop depends on 3 interrelated surface tensions and is determined using Young′s equation (see Figure 2.9.45.-2, 1st part):
γS | = | surface tension of the solid with air; |
γSL | = | interfacial tension of the solid with the liquid; |
γL | = | surface tension of the liquid with air. |
PROCEDURE
Since powders are unable to form a completely flat surface, the powder is usually compacted as a disc in an attempt to make the surface smoother. A liquid drop with a given volume is placed on the disc (see Figure 2.9.45.-2) allowing direct measurement of the contact angle using a goniometer fitted with an eyepiece protractor, or by geometric construction on a photomicrograph. Other physical and mathematical procedures of data analysis may also be appropriate. The drop volume may influence the result. Several determinations of the contact angle (θ) (n = 6) are usually carried out and the average is calculated.
WASHBURN METHOD
The Washburn method is able to measure the contact angle of porous solids with a contact angle in the range of 0-90°.
The tested material is the combination of the sample, the holder and the filter system. Therefore, an estimation or determination of the true value is not possible and only apparent values of the contact angle can be determined. However, the contact angle of the sample is the functional property on which the result is significantly dependent. The outcome of the test is a ranking order listing the wettability of different substances or formulations characterised by an apparent contact angle.
PRINCIPLE
If a porous solid is brought into contact with a liquid, such that the solid is not submerged in the liquid, but rather is just touching the liquid surface, then the rise of liquid into the pores of the solid due to capillary action will be governed by the following equations:
m | = | mass of liquid sucked into the solid; |
t | = | time elapsed since the solid and the liquid were brought into contact; |
A | = | constant, dependent on the properties of the liquid and the solid to be examined, calculated using the following equation: |
η | = | viscosity of the liquid; |
ρ | = | density of the liquid; |
γ | = | surface tension of the liquid; |
θ | = | contact angle between the solid and the liquid; |
c | = | material constant, dependent on the porous texture of the solid. |
Equations (1) and (2) lead to equation (3):
In setting up a Washburn determination, a liquid with known density (ρ), viscosity (η), and surface tension (γ) is used. Under these conditions, when the mass of liquid rising into the porous solid is monitored as a function of time (such that capillary penetration rate 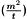 is the experimental data), 2 unknowns remain according to equation (3): the contact angle (θ) of the liquid on the solid, and the solid material constant (c).
is the experimental data), 2 unknowns remain according to equation (3): the contact angle (θ) of the liquid on the solid, and the solid material constant (c).
Determination of the material constant (c)
The material constant for a porous solid is determined by the following equation, considering cylindrical pores:
r | = | average capillary radius within the porous solid; |
N | = | number of capillaries per volumetric unit. |
If a Washburn determination is performed with a liquid considered to have a contact angle of 0° (cos 0° = 1) on the solid, then the solid material constant (c) is the only remaining unknown in equation (3) and can thus be determined. n-Heptane is the liquid of choice for determining material constants because of its low surface tension (20.14 mN⋅m-1 at 25 °C). n-Hexane may also be used (18.43 mN⋅m-1 at 25 °C) but is more volatile. If the powder dissolves too quickly in these liquids, hexamethyldisiloxane may be used instead (15.9 mN⋅m-1 at 25 °C). Replicate determinations are performed (n = 6) and the average value calculated.
Once the material constant (c) has been determined for the solid to be examined, a sample of the solid can be tested for wettability by another liquid. The material constant determined by the n-heptane test is used in the Washburn equation, in combination with the capillary penetration rate 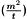 data obtained while testing the substance to be examined in the prescribed liquid. This allows calculation of the contact angle.
data obtained while testing the substance to be examined in the prescribed liquid. This allows calculation of the contact angle.
NOTE: if a series of liquids (at least 2 liquids in addition to the liquid used to determine the material constant) is tested against a given solid then the resultant contact angle data can be used to calculate the surface energy of the porous solid.
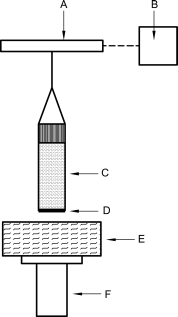
APPARATUS
Figure 2.9.45.-3 shows the principal components of the apparatus. The main device is an electronic balance with a suitable processor ensuring a suitable resolution in force measurement and a suitable resolution in lifting up the immersion liquid towards the sample.
A. electronic balance | C. sample holder | E. immersion liquid | |
B. computer | D. filter | F. lift |
Table 2.9.45.-1 indicates parameters of the electronic balance that are generally considered suitable.
Sample holders
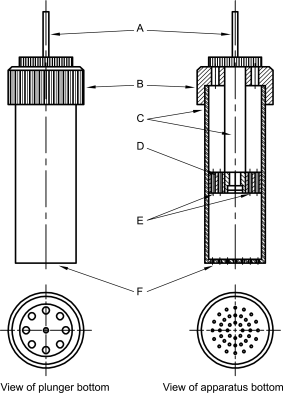
The sample holder may be a small glass cylinder with a sintered-glass filter at one end.
Powder material holders (see Figure 2.9.45-4) may also be made of aluminium; they are less fragile than those made of glass and have small holes in the bottom that render them easier to clean than a sintered-glass filter. The cover for the cell is equipped with 2 screw threads. One connects it with the sample chamber while the other allows the user to guide a piston down onto the sample itself and compact it. The apparatus is similar to an automatic tensiometer, except for the sample holder.
A. fixing | C. thread | E. capillary holes | |
B. cover | D. plunger | F. capillary holes |
procedure
Filling of the sample holder
Place a disc of filter paper in the bottom of the aluminium or glass sample holder. This prevents powder from leaking out of the bottom of the cell. The filter does not have to be made of paper, but it must be a material that is easily wetted by the liquid to be tested. A black-band filter (used for reverse osmosis) is recommended because of its high porosity and minimum flow resistance.
Place a known amount of powder into the cell. The reproducibility of material constants and contact angles will depend on the ability to weigh out the same amount of powder for each test when a sufficient and adjusted amount of powder is compacted in a uniform way (i.e. tapping/compaction of the powder).
For most powders, a correct amount is in the range of a few grams, typically filling about 2/3 of the capacity of the holder. Place a second piece of filter paper on top of the powder in the cell. This will prevent powder from rising through the holes in the piston during the compaction process and/or during the determination.
Tapping/compaction of the powder
A bulk powder bed is very porous and thus very sensitive to small influences that can easily alter the porosity and consequently the c-constant. Therefore a tapped powder may be advantageous and will show more reproducible results. The appropriate number of taps must first be evaluated: 50-100 taps are usually appropriate.
If the aluminium sample holder is used then it may be mounted in the cylinder of a stamp volumeter, which can run the evaluated number of taps.
If tapping is not appropriate, the powder bed is compacted by screwing the piston of the aluminium sample holder applying a specified pressure.
A further possibility is centrifugation under defined conditions. Where applicable, a compacted disc of the powder sample may also be mounted on the electronic balance. A sample holder is omitted in this case.
After connecting to the balance, the sample holder is positioned with the porous solid just above the surface of the liquid (see Figure 2.9.45.-3), using the lift.
The liquid is raised further until it just touches the bottom of the porous sample. Mass-versus-time data is then collected as liquid penetrates into the solid. Data can be presented in either graphical or tabular format. The apparatus may perform the whole determination automatically.
CRITICAL PARAMETERS
The following points must be considered.