Appendix XV J (Vet) 1. Cell Cultures for the Production of Veterinary Vaccines
Cell cultures for the production of vaccines for veterinary use comply with the requirements of this section. It may also be necessary that cell cultures used for testing of vaccines for veterinary use also comply with some or all of these requirements.
For most mammalian viruses, propagation in cell lines is possible and the use of primary cells is then not acceptable.
Permanently infected cells used for production of veterinary vaccines comply with the appropriate requirements described below. The cells shall be shown to be infected only with the agent stated.
CELL LINES
Cell lines are normally handled according to a cell-seed system. Each master cell seed is assigned a specific code for identification purposes. The master cell seed is stored in aliquots at – 70 °C or lower. Production of vaccine is not normally undertaken on cells more than twenty passages from the master cell seed. Where suspension cultures are used, an increase in cell numbers equivalent to approximately three population doublings is considered equivalent to one passage. If cells beyond twenty passage levels are to be used for production, it shall be demonstrated, by validation or further testing, that the production cell cultures are essentially similar to the master cell seed with regard to their biological characteristics and purity and that the use of such cells has no deleterious effect on vaccine production.
The history of the cell line shall be known and recorded in detail (for example, origin, number of passages and media used for multiplication, storage conditions).
The method of storing and using the cells, including details of how it is ensured that the maximum number of passages permitted is not exceeded during product manufacture, are recorded. A sufficient quantity of the master cell seed and each working cell seed are kept for analytical purposes.
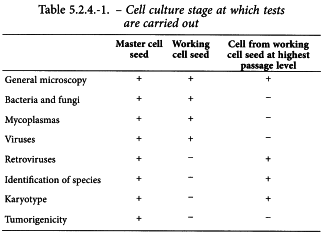
The tests described below are carried out (as prescribed in Table 5.2.4.-1) on a culture of the master cell seed and the working cell seed or on cell cultures from the working cell seed at the highest passage level used for production and derived from a homogeneous sample demonstrated to be representative.
Characteristics of culture
The appearance of cell monolayers, before and after histological staining, is described. Information, if possible numerical data, is provided especially on the speed and rate of growth. Similarly, the presence or absence of contact inhibition, polynucleated cells and any other cellular abnormalities are specified.
Karyotype
A chromosomal examination is made of not fewer than fifty cells undergoing mitosis in the master cell seed and at a passage level at least as high as that to be used in production. Any chromosomal marker present in the master cell seed must also be found in the high passage cells and the modal number of chromosomes in these cells must not be more than 15 per cent higher than of cells of the master cell seed. The karyotypes must be identical. If the modal number exceeds the level stated, if the chromosomal markers are not found in the working cell seed at the highest level used for production or if the karyotype differs, the cell line shall not be used for manufacture.
Identification of the species
It shall be shown, by one validated method, that the master cell seed and the cells from the working cell seed at the highest passage level used for production come from the species of origin specified. When a fluorescence test is carried out and the corresponding serum to the species of origin of cells is used and shows that all the tested cells are fluorescent, it is not necessary to carry out other tests with reagents able to detect contamination by cells of other species.
Bacterial and fungal contamination
The cells comply with the test for sterility (2.6.1). The sample of cells to be examined consists of not less than the number of cells in a monolayer with an area of 70 cm2 or, for cells grown in suspension, an approximately equivalent number of cells. The cells are maintained in culture for at least 15 days without antibiotics before carrying out the test.
Mycoplasmas (2.6.7)
The cells comply with the test for mycoplasmas. The cells are maintained in culture for at least 15 days without antibiotics before carrying out the test.
Absence of contaminating viruses
The cells must not be contaminated by viruses; suitably sensitive tests, including those prescribed below, are carried out.
The monolayers tested shall have an area of at least 70 cm2, and shall be prepared and maintained using medium and additives, and grown under similar conditions to those used for the preparation of the vaccine. The monolayers are maintained in culture for a total of at least 28 days. Subcultures are made at 7-day intervals, unless the cells do not survive for this length of time, when the subcultures are made on the latest day possible. Sufficient cells, in suitable containers, are produced for the final subculture to carry out the tests specified below.
The monolayers are examined regularly throughout the incubation period for the possible presence of cytopathic effects and at the end of the observation period for cytopathic effects, haemadsorbent viruses and specific viruses by immuno-fluorescence and other suitable tests as indicated below.
Detection of cytopathic viruses
Two monolayers of at least 6 cm2 each are stained with an appropriate cytological stain. The entire area of each stained monolayer is examined for any inclusion bodies, abnormal numbers of giant cells or any other lesion indicative of a cellular abnormality which might be attributable to a contaminant.
Detection of haemadsorbent viruses
Monolayers totalling at least 70 cm2 are washed several times with an appropriate buffer and a sufficient volume of a suspension of suitable red blood cells added to cover the surface of the monolayer evenly. After different incubation times cells are examined for the presence of haemadsorption.
Detection of specified viruses
Tests are carried out for freedom from contaminants specific for the species of origin of the cell line and for the species for which the product is intended. Sufficient cells on suitable supports are prepared to carry out tests for the agents specified. Suitable positive controls are included in each test. The cells are subjected to suitable tests, for example using fluorescein-conjugated antibodies or similar reagents.
Tests in other cell cultures
Monolayers totalling at least 140 cm2 are required. The cells are frozen and thawed at least three times and then centrifuged to remove cellular debris. Inoculate aliquots onto the following cells at any time up to 70 per cent confluency:
The inoculated cells are maintained in culture for at least 7 days, after which freeze-thawed extracts are prepared as above and inoculated onto sufficient fresh cultures of the same cell types to allow for the testing as described below. The cells are incubated for at least a further 7 days. The cultures are examined regularly for the presence of any cytopathic changes indicative of living organisms.
At the end of this period of 14 days, the inoculated cells are subjected to the following checks:
Retroviruses
A validated in vitro test is carried out to detect the presence of retroviruses in cell lines. If the presence of retrovirus is known or established by testing such as an endpoint product-enhanced reverse transcriptase (PERT) assay (2.6.21), then infectivity assays should be carried out. A PERT assay may be suitable to detect infective retrovirus after passage on permissive cells.
Since the sensitivity of PERT assays is very high, interpretation of a positive signal may be equivocal.
Cell seeds that show the presence of infectious retroviruses are not acceptable for the production of vaccines. In exceptional cases of positive or equivocal result in the infectivity assay, it may be justified and authorised to use such cells. Such a justification is based on a risk assessment including all available data and any down stream processing steps until the final product stage. The results of the risk assessment must demonstrate that the risk associated with the presence of infectious retroviruses is negligible in the final product.
Tumorigenicity
The risk of a cell line for the target species must be evaluated and, if necessary, tests are carried out.
PRIMARY CELLS
For most mammalian vaccines, the use of primary cells is not acceptable for the manufacture of vaccines since cell lines can be used. If there is no alternative to the use of primary cells, the cells are obtained from a herd or flock free from specified pathogens, with complete protection from introduction of diseases (for example, disease barriers, filters on air inlets, suitable quarantine before introduction of animals). Chicken flocks comply with the requirements prescribed in general chapter 5.2.2. Chicken Flocks Free from Specified Pathogens for the Production and Quality Control of Vaccines. For all other species, the herd or flock is shown to be free from relevant specified pathogens. All the breeding stock in the herd or flock intended to be used to produce primary cells for vaccine manufacture is subject to a suitable monitoring procedure including regular serological checks carried out at least twice a year and two supplementary serological examinations performed in 15 per cent of the breeding stock in the herd between the two checks mentioned above.
Wherever possible, particularly for mammalian cells, a seed-lot system is used with, for example, a master cell seed formed after less than five passages, the working cell seed being no more than five passages from the initial preparation of the cell suspension from the animal tissues.
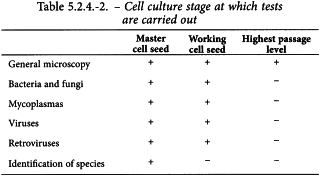
Each master cell seed, working cell seed and cells of the highest passage of primary cells are checked in accordance with Table 5.2.4.-2 and the procedure described below. The sample tested shall cover all the sources of cells used for the manufacture of the batch. No batches of vaccine manufactured using the cells may be released if any one of the checks performed produces unsatisfactory results.
Characteristics of cultures
The appearance of cell monolayers, before and after histological staining, is described. Information, if possible numerical data, is recorded, especially on the speed and rate of growth. Similarly, the presence or absence of contact inhibition, polynucleated cells and any other cellular abnormalities are specified.
Identification of species
It shall be demonstrated by one validated test that the master cell seed comes from the specified species of origin.
When a fluorescence test is carried out and the corresponding serum to the species of origin of cells is used and shows that all the tested cells are fluorescent, it is not necessary to carry out other tests with reagents able to detect contamination by cells of other species.
Bacterial and fungal sterility
The cells comply with the test for sterility (2.6.1). The sample of cells to be examined consists of not less than the number of cells in a monolayer with an area of 70 cm2 or for cells grown in suspension an approximately equivalent number of cells. The cells are maintained in culture for at least 15 days without antibiotics before carrying out the test.
Mycoplasmas (2.6.7)
The cells comply with the test for mycoplasmas. The cells are maintained in culture for at least 15 days without antibiotics before carrying out the test.
Absence of contaminating viruses
The cells must not be contaminated by viruses; suitably sensitive tests, including those prescribed below are carried out.
The monolayers tested shall be at least 70 cm2, and shall be prepared and maintained in culture using the same medium and additives, and under similar conditions to those used for the preparation of the vaccine.
The monolayers are maintained in culture for a total of at least 28 days or for the longest period possible if culture for 28 days is impossible. Subcultures are made at 7-day intervals, unless the cells do not survive for this length of time when the subcultures are made on the latest day possible. Sufficient cells, in suitable containers are produced for the final subculture to carry out the tests specified below.
The monolayers are examined regularly throughout the incubation period for the possible presence of cytopathic effects and at the end of the observation period for cytopathic effects, haemadsorbent viruses and specific viruses by immunofluorescence and other suitable tests as indicated below.
Detection of cytopathic viruses
Two monolayers of at least 6 cm2 each are stained with an appropriate cytological stain. Examine the entire area of each stained monolayer for any inclusion bodies, abnormal numbers of giant cells or any other lesion indicative of a cellular abnormality that might be attributable to a contaminant.
Detection of haemadsorbent viruses
Monolayers totalling at least 70 cm2 are washed several times with a suitable buffer solution and a sufficient volume of a suspension of suitable red blood cells added to cover the surface of the monolayer evenly. After different incubation times, examine cells for the presence of haemadsorption.
Detection of specified viruses
Tests are be carried out for freedom of contaminants specific for the species of origin of the cells and for the species for which the product is intended.
Sufficient cells on suitable supports are prepared to carry out tests for the agents specified. Suitable positive controls are included in each test. The cells are subjected to suitable tests using fluorescein-conjugated antibodies or similar reagents.
Tests in other cell cultures
Monolayers totalling at least 140 cm2 are required. The cells are frozen and thawed at least three times and then centrifuged to remove cellular debris. Aliquots are inoculated onto the following cells at any time up to 70 per cent confluency:
The inoculated cells are maintained in culture for at least 7 days, after which freeze-thawed extracts are prepared as above, and inoculated onto sufficient fresh cultures of the same cell types to allow for the testing as described below. The cells are incubated for at least a further 7 days. All cultures are regularly examined for the presence of any cytopathic changes indicative of living organisms.
At the end of this period of 14 days, the inoculated cells are subjected to the following checks:
Retroviruses
A validated in vitro test is carried out to detect the presence of retroviruses in primary cells. If the presence of retrovirus is known or established by testing such as an endpoint product-enhanced reverse transcriptase (PERT) assay (2.6.21), then infectivity assays should be carried out. A PERT assay may be suitable to detect infective retrovirus after passage on permissive cells.
Since the sensitivity of PERT assays is very high, interpretation of a positive signal may be equivocal.
Primary cells that show the presence of infectious retroviruses are not acceptable for the production of vaccines. In exceptional cases of positive or equivocal result in the infectivity assay, it may be justified and authorised to use such cells. Such a justification is based on a risk assessment including all available data and any down stream processing steps until the final product stage. The results of the risk assessment must demonstrate that the risk associated with the presence of infectious retroviruses is negligible in the final product.