Appendix XVI B (Vet) 4. Avian Viral Vaccines: Tests for Extraneous Agents in Seed Lots
GENERAL provisions
1 TEST FOR EXTRANEOUS AGENTS USING EMBRYONATED HENS’ EGGS
Use a test substance, diluted if necessary, containing a quantity of neutralised virus equivalent to at least 10 doses of vaccine in 0.2 mL of inoculum. Suitable antibiotics may be added. Inoculate the test substance into 3 groups of 10 embryonated hens’ eggs as follows:
Candle the eggs in groups 1 and 2 daily for 7 days and the eggs in group 3 daily for 12 days. Discard embryos that die during the first 24 h as non-specific deaths; the test is not valid unless at least 6 embryos in each group survive beyond the first 24 h after inoculation. Examine macroscopically for abnormalities all embryos that die more than 24 h after inoculation, or that survive the incubation period. Examine also the chorio-allantoic membranes of these eggs for any abnormality and test the allantoic fluids for the presence of haemagglutinating agents.
Carry out a further embryo passage. Pool separately material from live and from the dead and abnormal embryos. Inoculate each pool into 10 eggs for each route as described above, chorio-allantoic membrane material being inoculated onto chorio-allantoic membranes, allantoic fluids into the allantoic cavity and embryo material into the yolk sac. For eggs inoculated by the allantoic and chorio-allantoic routes, candle the eggs daily for 7 days, proceeding and examining the material as described above. For eggs inoculated by the yolk sac route, candle the eggs daily for 12 days, proceeding and examining the material as described above.
The seed lot complies with the test if no test embryo shows macroscopic abnormalities or dies from causes attributable to the seed lot and if examination of the chorio-allantoic membranes and testing of the allantoic fluids show no evidence of the presence of any extraneous agent.
2 TEST IN CHICKEN KIDNEY CELLS
Prepare 7 monolayers of chicken kidney cells, each monolayer having an area of about 25 cm2. Maintain 2 monolayers as negative controls and treat these in the same way as the 5 monolayers inoculated with the test substance, as described below. Remove the culture medium when the cells reach confluence. Inoculate 0.1 mL of the test substance onto each of the 5 monolayers. Allow adsorption for 1 h, add culture medium and incubate the cultures for a total of at least 21 days, subculturing at 4- to 7-day intervals. Each passage is made with pooled cells and fluids from all 5 monolayers after carrying out a freeze-thaw cycle. Inoculate 0.1 mL of pooled material onto each of 5 recently prepared monolayers of about 25 cm2 each, at each passage. For the last passage, grow the cells also on a suitable substrate so as to obtain an area of about 10 cm2 of cells from each of the monolayers for test A. The test is not valid if less than 80 per cent of the monolayers survive after any passage.
Examine microscopically all the cell cultures frequently throughout the entire incubation period for any signs of cytopathic effect or other evidence of the presence of contaminating agents in the test substance. At the end of the total incubation period, carry out the following procedures.
The test is not valid if there are any signs of extraneous agents in the negative control cultures. The seed lot complies with the test if there is no evidence of the presence of any extraneous agent.
3 TEST FOR AVIAN LEUCOSIS VIRUSES
Prepare at least 13 replicate monolayers of either DF-1 cells or primary or secondary chick embryo fibroblasts from the tissues of 9- to 11-day-old embryos that are known to be genetically susceptible to subgroups A, B and J of avian leucosis viruses and that support the growth of exogenous but not endogenous avian leucosis viruses (cells from C/E strain chickens are suitable). Each replicate shall have an area of about 50 cm2.
Remove the culture medium when the cells reach confluence. Inoculate 0.1 mL of the test substance onto each of 5 of the replicate monolayers. Allow adsorption for 1 h, and add culture medium. Inoculate 2 of the replicate monolayers with subgroup A avian leucosis virus (not more than 10 CCID50 in 0.1 mL), 2 with subgroup B avian leucosis virus (not more than 10 CCID50 in 0.1 mL) and 2 with subgroup J avian leucosis virus (not more than 10 CCID50 in 0.1 mL) as positive controls. Maintain not fewer than 2 non-inoculated replicate monolayers as negative controls.
Incubate the cells for a total of at least 9 days, subculturing at 3- to 4-day intervals. Retain cells from each passage level and harvest the cells at the end of the total incubation period. Wash cells from each passage level from each replicate and resuspend the cells at 107 cells per millilitre in barbital-buffered saline for subsequent testing by a Complement Fixation for Avian Leucosis (COFAL) test or in phosphate buffered saline for testing by Enzyme-Linked Immunosorbent Assay (ELISA). Then, carry out 3 cycles of freezing and thawing to release any group-specific antigen and perform a COFAL test or an ELISA test on each extract to detect group-specific avian leucosis antigen if present.
The test is not valid if group-specific antigen is detected in fewer than 5 of the 6 positive control replicate monolayers or if a positive result is obtained in any of the negative control monolayers, or if the results for both of the 2 negative control monolayers are inconclusive. If the results for more than 1 of the test replicate monolayers are inconclusive, then further subcultures of reserved portions of the fibroblast monolayers shall be made and tested until an unequivocal result is obtained. If a positive result is obtained for any of the test monolayers, then the presence of avian leucosis virus in the test substance has been detected.
The seed lot complies with the test if there is no evidence of the presence of any avian leucosis virus.
4 TEST FOR AVIAN RETICULOENDOTHELIOSIS VIRUS
Prepare 11 monolayers of primary or secondary chick embryo fibroblasts from the tissues of 9- to 11-day old chick embryos or duck embryo fibroblasts from the tissues of 13- to 14-day-old embryos, each monolayer having an area of about 25 cm2.
Remove the culture medium when the cells reach confluence. Inoculate 0.1 mL of the test substance onto each of 5 of the monolayers. Allow adsorption for 1 h, and add culture medium. Inoculate 4 of the monolayers with avian reticuloendotheliosis virus as positive controls (not more than 10 CCID50 in 0.1 mL). Maintain 2 non-inoculated monolayers as negative controls.
Incubate the cells for a total of at least 10 days, subculturing twice at 3- to 4-day intervals. The test is not valid if fewer than 3 of the 4 positive controls or fewer than 4 of the 5 test monolayers or neither of the 2 negative controls survive after any passage.
For the last subculture, grow the fibroblasts on a suitable substrate so as to obtain an area of about 10 cm2 of confluent fibroblasts from each of the original 11 monolayers for the subsequent test: test about 10 cm2 of confluent fibroblasts derived from each of the original 11 monolayers by immunostaining for the presence of avian reticuloendotheliosis virus. The test is not valid if avian reticuloendotheliosis virus is detected in fewer than 3 of the 4 positive control monolayers or in any of the negative control monolayers, or if the results for both of the 2 negative control monolayers are inconclusive. If the results for more than 1 of the test monolayers are inconclusive then further subcultures of reserved portions of the fibroblast monolayers shall be made and tested until an unequivocal result is obtained.
The seed lot complies with the test if there is no evidence of the presence of avian reticuloendotheliosis virus.
5 TEST FOR CHICKEN ANAEMIA VIRUS
Prepare eleven 20 mL suspensions of the MDCC-MSBI cell line or another cell line of equivalent sensitivity in 25 cm2 cell culture flasks containing about 5 × 105 cells/mL. Inoculate 0.1 mL of the test substance into each of 5 flasks. Inoculate 4 of the suspensions with 10 CCID50 chicken anaemia virus as positive controls. Maintain not fewer than 2 non-inoculated suspensions. Maintain all the cell cultures for a total of at least 24 days, subculturing 8 times at 3- to 4-day intervals. During the subculturing the presence of chicken anaemia virus may be indicated by a metabolic colour change in the infected cultures, the culture fluids becoming red in comparison with the control cultures. Examine the cells microscopically for cytopathic effect. At this time or at the end of the incubation period, centrifuge the cells from each flask at low speed and resuspend at about 106 cells/mL and place 25 µL in each of 10 wells of a multi-well slide. Examine the cells by immunostaining.
The test is not valid if chicken anaemia virus is detected in fewer than 3 of the 4 positive controls or in any of the non-inoculated controls. If the results for more than 1 of the test suspensions are inconclusive, then further subcultures of reserved portions of the test suspensions shall be made and tested until an unequivocal result is obtained.
The seed lot complies with the test if there is no evidence of the presence of chicken anaemia virus.
6 TEST FOR EXTRANEOUS AGENTS USING CHICKS
Inoculate each of at least 10 chicks with the equivalent of 100 doses of vaccine by the intramuscular route and with the equivalent of 10 doses by eye-drop. Chicks that are 2 weeks of age are used in the test except that if the seed virus is pathogenic for birds of this age, older birds may be used, if required and justified. In exceptional cases, for inactivated vaccines, the virus may be neutralised by specific antiserum if the seed virus is pathogenic for birds at the age of administration. Repeat these inoculations 2 weeks later. Observe the chicks for a period of 5 weeks from the day of the first inoculation. No antimicrobial agents shall be administered to the chicks during the test period. The test is not valid if fewer than 80 per cent of the chicks survive to the end of the test period.
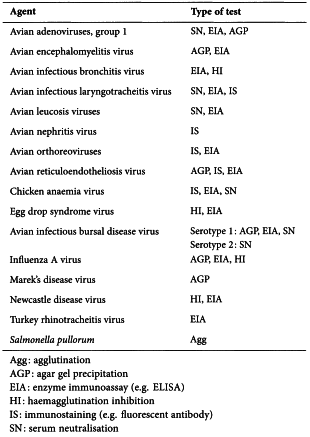
Collect serum from each chick at the end of the test period. Test each serum sample for antibodies against each of the agents listed below (with the exception of the virus type of the seed lot) using one of the methods indicated for testing for the agent.
Clinical signs of disease in the chicks during the test period (other than signs attributable to the virus of the seed lot) and the detection of antibodies in the chicks after inoculation (with the exception of antibodies to the virus of the seed lot), are classed as evidence of the presence of an extraneous agent in the seed lot.
It is recommended that sera from these birds is retained so that additional testing may be carried out if requirements change.
A. Standard tests
B. Additional tests for turkey extraneous agents
If the seed virus is of turkey origin or was propagated in turkey substrates, tests for antibodies against the following agents are also carried out.
A test for freedom from turkey lympho-proliferative disease virus is carried out by intraperitoneal inoculation of twenty 4-week-old turkey poults. Observe the poults for 40 days. The test is not valid if more than 20 per cent of the poults die from non-specific causes. The seed lot complies with the test if sections of spleen and thymus taken from 10 poults 2 weeks after inoculation show no macroscopic or microscopic lesions (other than those attributable to the seed lot virus) and no poult dies from causes attributable to the seed lot.
C. Additional tests for duck extraneous agents
If the seed virus is of duck origin or was propagated in duck substrates, tests for antibodies against the following agents are also carried out.
The seed lot complies with the test if there is no evidence of the presence of any extraneous agent.
D. Additional tests for goose extraneous agents
If the seed virus is of goose origin or was prepared in goose substrates, tests for the following agents are also carried out.
Inoculate subcutaneously the equivalent of at least 10 doses to each of ten 1-day-old susceptible goslings. Observe the goslings for 28 days. The test is not valid if more than 20 per cent of the goslings die from non-specific causes. The seed virus complies with the test if no gosling dies from causes attributable to the seed lot.
7 ANTIBODY SPECIFICATIONS FOR SERA USED IN EXTRANEOUS AGENTS TESTING
All batches of serum to be used in extraneous agents testing, either to neutralise the vaccine virus (seed lot or batch of finished product) or as a supplement for cell culture media, shall be shown by suitably sensitive tests to be free from antibodies against and free from inhibitory effects on the following micro-organisms - except for one type, namely, antibodies against the virus that they are supposed to neutralise.
Avian adenoviruses
Avian encephalomyelitis virus
Avian infectious bronchitis viruses
Avian infectious bursal disease virus types 1 and 2
Avian infectious haemorrhagic enteritis virus
Avian infectious laryngotracheitis virus
Avian leucosis viruses
Avian nephritis virus
Avian paramyxoviruses 1 to 9
Avian orthoreoviruses
Avian reticuloendotheliosis virus
Chicken anaemia virus
Duck enteritis virus
Duck hepatitis virus type I
Egg drop syndrome virus
Fowl pox virus
Influenza viruses
Marek’s disease virus
Turkey herpesvirus
Turkey rhinotracheitis virus
Non-immune serum for addition to culture media can be assumed to be free from antibodies against any of these viruses if the agent is known not to infect the species of origin of the serum and it is not necessary to test the serum for such antibodies. Monospecific antisera for virus neutralisation can be assumed to be free from the antibodies against any of these viruses if it can be shown that the immunising antigen could not have been contaminated with antigens derived from that virus and if the virus is known not to infect the species of origin of the serum; it is not necessary to test the serum for such antibodies. It is not necessary to retest sera obtained from birds from SPF chicken flocks (5.2.2).
Batches of sera prepared for neutralising the vaccine virus must not be prepared from any passage level derived from the virus isolate used to prepare the master seed lot or from an isolate cultured in the same cell line.