Aciclovir Tablets
Action and use
Purine nucleoside analogue; antiviral (herpesviruses).
Definition
Aciclovir Tablets contain Aciclovir.
Content of aciclovir, C8H11N5O3
95.0 to 105.0% of the stated amount.
Identification
TESTS
Dissolution
Comply with the requirements for Monographs of the British Pharmacopoeia in the dissolution test for tablets and capsules, Appendix XII B1.
After 45 minutes, withdraw a 25 mL sample of the medium and measure the absorbance of the filtered sample, suitably diluted with the dissolution medium if necessary, at the maximum at 255 nm, Appendix II B, using 0.1m hydrochloric acid in the reference cell.
Calculate the total content of aciclovir, C8H11N5O3, in the medium from the absorbance obtained and taking 560 as the value of A(1%, 1 cm) at the maximum at 255 nm.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in a solvent mixture of 1 volume of dimethyl sulfoxide and 4 volumes of water unless otherwise indicated.
Phosphate buffer solution pH 3.1Dissolve 3.48 g of dipotassium hydrogen orthophosphate in 1000 mL of water and adjust to pH 3.1 with orthophosphoric acid.
Phosphate buffer solution pH 2.5Dissolve 3.48 g of dipotassium hydrogen orthophosphate in 1000 mL of water and adjust to pH 2.5 with orthophosphoric acid.
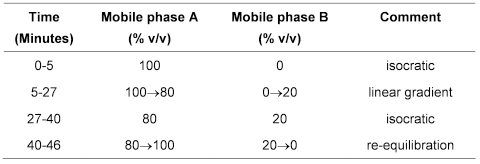
Mobile phase A1 volume of acetonitrile and 99 volumes of phosphate buffer solution pH 3.1.
Mobile phase B50 volumes of acetonitrile and 50 volumes of phosphate buffer solution pH 2.5.
The test is not valid unless:
in the chromatogram obtained with solution (4), the resolution between the peaks due to impurity C and aciclovir is at least 1.5;
in the chromatogram obtained with solution (5), the resolution between the peaks due to impurity F and impurity A is at least 1.5 and the resolution between the peaks due to impurity K and G is at least 1.5.
In the chromatogram obtained with solution (1):
identify any peak corresponding to impurity I using the chromatogram obtained with solution (4) and the chromatogram supplied with aciclovir for peak identification 1 EPCRS and multiply the area of this peak by a correction factor of 1.5;
the area of any peak corresponding to impurity B is not greater than 5 times the area of the principal peak in the chromatogram obtained with solution (2) (1.0%);
the area of any peak corresponding to impurity O is not greater than 1.5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.3%);
the area of any peak corresponding to impurity A is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.2%);
the area of any other secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.2%);
the sum of the areas of all secondary peaks is not greater than 10 times the area of the principal peak in the chromatogram obtained with solution (2) (2.0%).
Disregard any peak with an area less than 0.25 times the area of the principal peak in the chromatogram obtained with solution (2) (0.05%).
Assay
Weigh and finely powder 20 tablets. Carry out the method for liquid chromatography, Appendix III D, using the following solutions in a solvent mixture of 1 volume of dimethyl sulfoxide and 4 volumes of water unless otherwise indicated.
The chromatographic conditions described under Related substances may be used.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between the peaks due to impurity C and aciclovir is at least 1.5.
Calculate the content of C8H11N5O3 in the tablets using the declared content of C8H11N5O3 in aciclovir BPCRS.
Impurities
The impurities limited by the requirements of this monograph include those listed under Aciclovir.