Carbimazole Tablets
Action and use
Thionamide antithyroid drug.
Definition
Carbimazole Tablets contain Carbimazole.
Content of carbimazole, C7H10N2O2S
90.0 to 110.0% of the stated amount.
Identification
Extract a quantity of the powdered tablets containing 50 mg of Carbimazole with two 5-mL quantities of dichloromethane. Combine the dichloromethane extracts, filter and evaporate the filtrate to dryness. The infrared absorption spectrum, Appendix II A, of the residue after drying at 60° at a pressure not exceeding 0.7 kPa for 30 minutes is concordant with the reference spectrum of carbimazole (RS 042).
Thiamazole and other related substances
Carry out the test protected from light and prepare the solutions immediately before use.
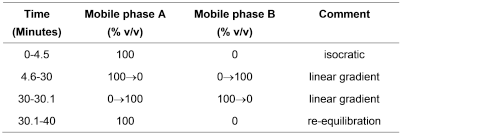
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
Mobile phase A5 volumes of acetonitrile and 95 volumes of water.
Mobile phase B20 volumes of acetonitrile and 80 volumes of water.
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution between the peaks due to carbimazole and thiamazole is at least 5.0.
In the chromatogram obtained with solution (1):
the area of any peak corresponding to thiamazole is not greater than the area of the principal peak in the chromatogram obtained with solution (3) (1%);
the area of any other secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.5%).
Assay
Carry out the test protected from light and prepare the solutions immediately before use.
Weigh and powder 20 tablets. Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
The chromatographic procedure described under Related substances may be used.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between the peaks due to carbimazole and thiamazole is at least 5.0.
Calculate the content of C7H10N2O2S in the tablets using the declared content of C7H10N2O2S in carbimazole BPCRS.
IMPURITIES
The impurities limited by the requirements of this monograph include those listed under Carbimazole.