Clomipramine Capsules
Action and use
Monoamine reuptake inhibitor; tricyclic antidepressant.
Definition
Clomipramine Capsules contain Clomipramine Hydrochloride.
Content of clomipramine hydrochloride, C19H23ClN2,HCl
95.0 to 105.0% of the stated amount.
Identification
TESTS
Related substances
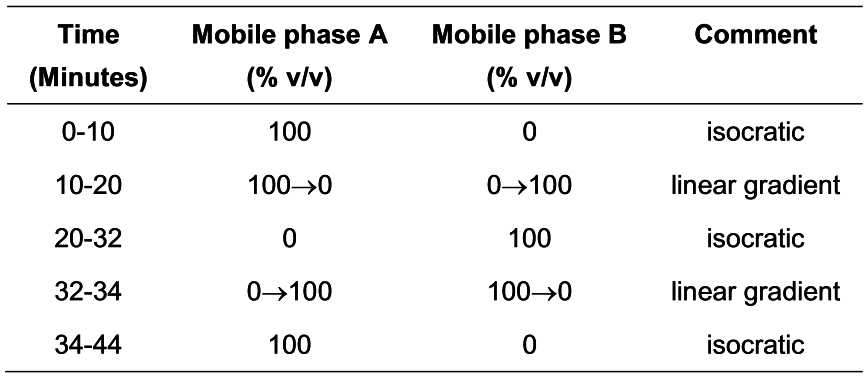
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
Mobile phase ADissolve 1.2 g of sodium dihydrogen orthophosphate in 950 mL of water, add 1.1 mL of nonylamine, adjust to pH 3.0 with orthophosphoric acid and add sufficient water to produce 1000 mL (solution A). Mix 75 volumes of solution A with 25 volumes of acetonitrile.
Mobile phase BMix 65 volumes of solution A and 35 volumes of acetonitrile.
The retention times relative to clomipramine are about 0.5, 0.7, 0.9, 1.7, 2.5, 3.4 and 4.3 for impurities A, B, C, D, E, F and G, respectively.
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution factor between the peaks due to clomipramine and clomipramine impurity C is at least 3.0.
In the chromatogram obtained with solution (1):
the area of any peak corresponding to clomipramine impurity B is not greater than the area of the corresponding peak in the chromatogram obtained with solution (3) (1%);
the area of any peak corresponding to impurity C or impurity D is not greater than the area of the corresponding peak in the chromatogram obtained with solution (3) (0.2%),
the area of any peak corresponding to impurity F is not greater than the area of the corresponding peak in the chromatogram obtained with solution (3) (0.2%);
the area of any other secondary peak is not greater than 0.2 times the area of the principal peak in the chromatogram obtained with solution (2) (0.2%);
and the sum of the areas of any such secondary peaks is not greater than 0.2 times the area of the principal peak in the chromatogram obtained with solution (2) (0.2%).
The sum of the impurities is not greater than 1.0%.
Disregard any peak with an area not greater than 0.01 times the area of the principal peak in the chromatogram obtained with solution (2) (0.01%).
Assay
Shake a quantity of the mixed contents of 20 capsules containing 50 mg of Clomipramine Hydrochloride with 200 mL of 0.1m hydrochloric acid for 1 hour, dilute to 250 mL with 0.1m hydrochloric acid and filter. Dilute 15 mL of the filtrate to 100 mL with 0.1m hydrochloric acid and measure the absorbance of the resulting solution at the maximum at 252 nm, Appendix II B. Calculate the content of C19H23ClN2,HCl taking 226 as the value of A(1%, 1 cm) at the maximum at 252 nm.