Domperidone Tablets
Action and use
Peripheral dopamine receptor antagonist; antiemetic.
Definition
Domperidone Tablets contain Domperidone Maleate.
Content of domperidone, C22H24ClN5O2
95.0 to 105.0% of the stated amount.
Identification
5 volumes of a solution prepared by dissolving 1.36 g of sodium acetate in 50 mL of water, adjusting the pH to 4.7 with dilute acetic acid and adding sufficient water to produce 100 mL, 18 volumes of methanol, 23 volumes of dichloromethane and 54 volumes of ethyl acetate.
Using each method of visualisation, the principal spot in the chromatogram obtained with solution (1) corresponds to that in the chromatogram obtained with solution (2).
TESTS
Dissolution
Comply with the requirements for Monographs of the British Pharmacopoeia in the dissolution test for tablets and capsules, Appendix XII B1.
Calculate the total content of domperidone, C22H24ClN5O2, in the medium from the absorbances obtained and using the declared content of C22H24ClN5O2, in domperidone maleate BPCRS.
Related substances
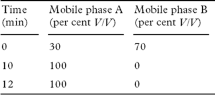
Carry out the method for liquid chromatography, Appendix III D, using the following solutions prepared immediately before use.
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution between the two principal peaks is at least 2.
In the chromatogram obtained with solution (1):
the area of any secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.25%);
the sum of the areas of any secondary peaks is not greater than twice the area of the principal peak in the chromatogram obtained with solution (2) (0.5%).
Disregard any peak in the chromatogram obtained with the blank solution, any peak due to maleic acid near the start of the chromatogram and any peak with an area less than the area of the peak in the chromatogram obtained with solution (3) (0.05%).
Assay
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
The chromatographic procedure described under Related substances may be used.
Calculate the content of C22H24ClN5O2 in the tablets using the declared content of C22H24ClN5O2 in domperidone maleate BPCRS.
Storage
Domperidone Tablets should be stored in an airtight container.
Labelling
The quantity of the active ingredient is stated in terms of the equivalent amount of domperidone.