Estradiol and Norethisterone Acetate Tablets
Action and use
Estrogen.
Definition
Estradiol and Norethisterone Acetate Tablets contain Estradiol Hemihydrate and Norethisterone Acetate. They are coated.
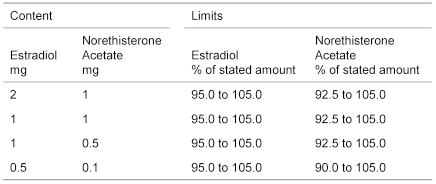
Content of estradiol, C18H24O2 and norethisterone acetate, C22H28O3
Identification
10 volumes of acetone and 90 volumes of dichloromethane.
The test is not valid unless the chromatogram obtained with solution (1) shows two clearly separated spots.
The two principal spots in the chromatogram obtained with solution (1) correspond in position and colour to those in the chromatogram obtained with solution (2).
Tests
Dissolution
Comply with the requirements for Monographs of the British Pharmacopoeia in the dissolution test for tablets and capsules, Appendix XII B1.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
450 volumes of water and 550 volumes of acetonitrile.
The test is not valid unless, in the chromatogram obtained with solution (2), the resolution factor between each pair of peaks (estradiol and norethisterone, and estrone and norethisterone acetate) is at least 1.0.
Calculate the total content of estradiol, C18H24O2, and of norethisterone acetate, C22H28O3, in the medium using the declared content of C18H24O2 in estradiol hemihydrate BPCRS and the declared content of C22H28O3 in norethisterone acetate BPCRS.
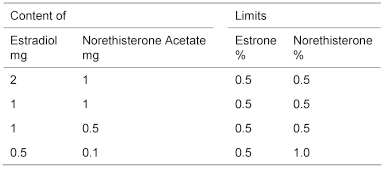
Estrone and norethisterone
Weigh and powder 20 tablets. Carry out the method for liquid chromatography, Appendix III D, using the following solutions in mobile phase.
The chromatographic conditions described under Dissolution may be used but injecting 20 µL of each solution.
The test is not valid unless, in the chromatogram obtained with solution (5), the resolution factor between each pair of peaks (estradiol and norethisterone, and estrone and norethisterone acetate) is at least 1.0.
In the chromatogram obtained with solution (1) quantify the area of any peak due to estrone using the principal peak in the chromatogram obtained with solution (3).
In the chromatogram obtained with solution (2) quantify the area of any peak due to norethisterone using the principal peak in the chromatogram obtained with solution (4).
Uniformity of content
Tablets containing less than 2 mg and/or less than 2% w/w of estradiol or less than 2 mg and/or less than 2% w/w of Norethisterone Acetate comply with the requirements stated under Tablets using the following method of analysis. Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
The chromatographic conditions described under Dissolution may be used but injecting 20 µL of each solution.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between each pair of peaks (estradiol and norethisterone, and estrone and norethisterone acetate) is at least 1.0.
Calculate the content of C18H24O2 and of C22H28O3 in each tablet using the declared content of C18H24O2 in estradiol hemihydrate BPCRS and the declared content of C22H28O3 in norethisterone acetate BPCRS.
Assay
For estradiol
For tablets containing less than 2 mg and/or less than 2% w/w of estradiol
Use the average of the 10 individual results obtained in the test for Uniformity of content.
For tablets containing 2 mg or more and 2% w/w or more of estradiol
Weigh and powder 20 tablets. Carry out the method for liquid chromatography, Appendix III D, using the following solutions in the mobile phase.
The chromatographic conditions described under Dissolution may be used but injecting 20 µL of each solution.
The test is not valid unless, in the chromatogram obtained with solution (5), the resolution factor between each pair of peaks (estradiol and norethisterone, and estrone and norethisterone acetate) is at least 1.0.
Calculate the content of C18H24O2 in each tablet using the declared content of C18H24O2 in estradiol hemihydrate BPCRS.
For norethisterone acetate
For tablets containing less than 2 mg and/or less than 2% w/w of estradiol
Use the average of the individual results determined in the test for Uniformity of content.
Storage
Estradiol and Norethisterone Acetate Tablets should be protected from light.
Labelling
The label states the quantity of estradiol hemihydrate in terms of the equivalent amount of estradiol.
Impurities
The impurities limited by the requirements of this monograph include: