Mercaptopurine Tablets
Action and use
Thiopurine cytotoxic.
Definition
Mercaptopurine Tablets contain Mercaptopurine.
Content of mercaptopurine, C5H4N4S,H2O
90.0 to 105.0% of the stated amount.
Carry out all of the following procedures protected from light.
Identification
Shake a quantity of the powdered tablets containing 50 mg of Mercaptopurine with 20 mL of absolute ethanol, filter and evaporate to dryness under reduced pressure. The infrared absorption spectrum of the residue, Appendix II A, is concordant with the reference spectrum of mercaptopurine (RS 426).
Tests
Dissolution
Comply with the requirements for Monographs of the British Pharmacopoeia in the dissolution test for tablets and capsules, Appendix XII B1, using the following conditions.
Withdraw a sample of 10 mL of the medium and filter. Measure the absorbance of the filtrate, Appendix II B, diluted with the dissolution medium, if necessary, at the maximum at 325 nm using dissolution medium in the reference cell. Measure the absorbance of a suitable solution of mercaptopurine BPCRS in the dissolution medium using the declared content of C5H4N4S,H2O in mercaptopurine BPCRS.
Related substances
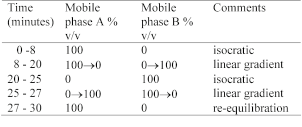
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
The test is not valid unless there is baseline resolution between the principal peaks corresponding to thioguanine, hypoxanthine and adenine in the chromatogram obtained with solution (5).
In the chromatogram obtained with solution (1):
the area of any peak corresponding to hypoxanthine is not greater than the area of the principal peak in the chromatogram obtained with solution (3) (2.0%);
the area of any peak corresponding to bis-(1,7-dihydro-6H-purine)-6,6-disulfide is not greater than the area of the principal peak in the chromatogram obtained with solution (4) (2.5%);
the area of any other secondary peak is not greater than twice the area of the principal peak in the chromatogram obtained with solution (2) (0.2%);
the sum of the areas of any other secondary peaks is not greater than 5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.5%).
Disregard any peak with an area less than 0.5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.05%).
Assay
Weigh and powder 20 tablets. Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
The chromatographic conditions described under Related substances may be used but with isocratic elution and the mobile phase described below.
2 volumes of methanol and 98 volumes of 0.1% v/v of anhydrous formic acid.
The test is not valid unless there is baseline resolution between the principal peaks corresponding to thioguanine, hypoxanthine and adenine in the chromatogram obtained with solution (5).
Calculate the total content of mercaptopurine, C5H4N4S,H2O, in the tablets using the chromatogram obtained and the declared content of C5H4N4S,H2O in mercaptopurine BPCRS.
Storage
Mercaptopurine Tablets should be protected from light.