Nabumetone Oral Suspension
Action and use
Cyclo-oxygenase inhibitor; analgesic; anti-inflammatory.
Definition
Nabumetone Oral Suspension contains Nabumetone in a suitable flavoured vehicle.
Content of nabumetone, C15H16O2
95.0 to 105.0% of the stated amount.
Identification
The principal spot in the chromatogram obtained with solution (1) is similar in position and colour to the spot in the chromatogram obtained with solution (2) and the chromatogram obtained with solution (3) shows a single, compact spot at the same Rf value as the spot in the chromatogram obtained with solution (2).
Related substances
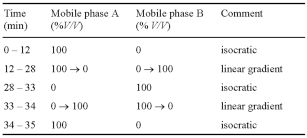
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
When the chromatograms are recorded under the prescribed conditions the retention time of nabumetone is about 11 minutes.
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution factor between the two principal peaks is at least 1.5.
In the chromatogram obtained with solution (1):
the area of any peak corresponding to nabumetone impurity F is not greater than the area of the principal peak in the chromatogram obtained with solution (3) (0.3%);
the sum of the areas of any other secondary peaks is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.5%).
Disregard any peak with an area less than 0.1 times the area of the principal peak in the chromatogram obtained with solution (2) (0.05%).
Assay
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
When the chromatograms are recorded under the prescribed conditions the retention time of nabumetone is about 4 minutes.
18 volumes of tetrahydrofuran, 40 volumes of a 0.1% v/v solution of glacial acetic acid in carbon dioxide-free water and 42 volumes of acetonitrile for chromatography.
Determine the weight per mL of the oral suspension, Appendix V G, and calculate the content of C15H16O2, weight in volume, from the chromatograms obtained using the declared content of C15H16O2 in nabumetone BPCRS.