Sulfasalazine Tablets
Action and use
Sulfonamide aminosalicylate; treatment of ulcerative colitis.
Definition
Sulfasalazine Tablets contain Sulfasalazine.
Content of sulfasalazine, C18H14N4O5S
95.0 to 105.0% of the stated amount.
Identification
Tests
Dissolution
Comply with the requirements for Monographs of the British Pharmacopoeia in the dissolution test for tablets and capsules, Appendix XII B1.
Calculate the total content of sulfasalazine, C18H14N4O5S, in the medium from the absorbances obtained and using the declared content of C18H14N4O5S, in sulfasalazine BPCRS.
Related substances
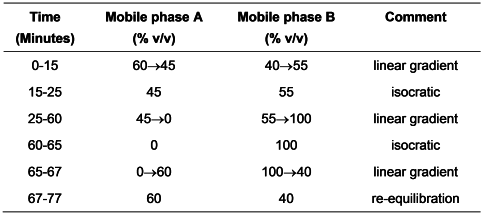
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
Mobile phase A0.013% w/v of sodium dihydrogen orthophosphate and 0.25% w/v of sodium acetate adjusted to pH 4.8 with glacial acetic acid.
Mobile phase B1 volume of mobile phase A and 4 volumes of methanol.
When the chromatograms are recorded in the prescribed conditions, the retention times relative to sulfasalazine (retention time about 24 minutes) are: salicylic acid (impurity H), about 0.16; impurity I, about 0.28; impurity C, about 0.80; impurity F, about 0.85; impurity G, about 1.39; impurity E, about 1.63; impurity B, about 1.85; impurity D, about 1.90 and impurity A, about 2.00.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between the peaks due to sulfasalazine and sulfasalazine derivative for resolution is at least 3.
In the chromatogram obtained with solution (1):
the area of any peaks corresponding to impurities A, B, C, D, E, F, G and I are not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1% of each);
the area of any other secondary peak is not greater than 0.1 times the area of the principal peak in the chromatogram obtained with solution (2) (0.1%);
the sum of the areas of any secondary peaks is not greater than four times the area of the principal peak in the chromatogram obtained with solution (2) (4%).
Disregard any peak with a retention time shorter than 6 minutes (corresponding to salicylic acid and sulfapyridine) and any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (5) (0.05%).
Salicylic acid and sulfapyridine
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
30 volumes of mobile phase B described in the test for Related substances and 70 volumes of mobile phase A described in the test for Related substances.
When the chromatogram is recorded using the prescribed conditions the retention time of salicylic acid is about 6 minutes and that of sulfapyridine is about 7 minutes.
The test is not valid unless, in the chromatogram obtained with solution (2), the resolution between the peaks due to salicylic acid and sulfapyridine is at least 2.
In the chromatogram obtained with solution (1), the area of any peak corresponding to salicylic acid or sulfapyridine is not greater than 0.5 times the area of the corresponding peaks in the chromatogram obtained with solution (2) (0.5% of each).
Assay
Weigh and powder 20 tablets. Shake a quantity of the powder containing 0.15 g of Sulfasalazine with about 50 mL of 0.1m sodium hydroxide, add sufficient of the same solvent to produce 100 mL, filter and discard the first 20 mL of filtrate. Dilute 5 mL of the filtrate with 750 mL of water, add 20 mL of 0.1m acetic acid, mix and add sufficient water to produce 1000 mL. Measure the absorbance of this solution at the maximum at 359 nm, Appendix II B. Calculate the content of C18H14N4O5S in the tablets from the absorbance of a standard solution prepared in the same manner using 0.15 g of sulfasalazine BPCRS and using the declared content of C18H14N4O5S in sulfasalazine BPCRS.
IMPURITIES
The impurities limited by the requirements of this monograph include those listed under Sulfasalazine.