Sulindac Tablets
Action and use
Cyclo-oxygenase inhibitor; analgesic; anti-inflammatory.
Definition
Sulindac Tablets contain Sulindac.
Content of sulindac, C20H17FO3S
95.0 to 105.0% of the stated amount.
Identification
Dissolution
Comply with the requirements for Monographs of the British Pharmacopoeia in the dissolution test for tablets and capsules, Appendix XII B1, using Apparatus 2. Use as the medium 900 mL of phosphate buffer pH 7.2 and rotate the paddle at 50 revolutions per minute. Withdraw a sample of 10 mL of the medium, filter and dilute with phosphate buffer pH 7.2 to give a solution expected to contain 0.001% w/v of Sulindac. Measure the absorbance of this solution, Appendix II B, at 327 nm using phosphate buffer pH 7.2 in the reference cell. Calculate the total content of C20H17FO3S in the medium taking 373 as the value of A(1%, 1 cm) at the maximum at 327 nm.
Related substances
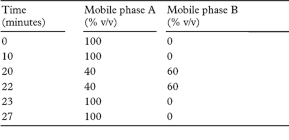
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. Mix 160 volumes of tetrahydrofuran and 840 volumes of methanol (solution A). Mix equal volumes of solution A and a solution containing 0.358% w/v of disodium hydrogen orthophosphate and 0.34% w/v of tetrabutylammonium hydrogen sulfate (the mixture is solution B). For solution (1) shake a quantity of whole tablets containing 1 g of Sulindac with 175 mL of solution A mechanically for 15 minutes and then with the aid of ultrasound until the tablets have fully disintegrated (at least 15 minutes). Allow the resulting suspension to cool, add sufficient of solution B to produce 200 mL and mix. Dilute 10 mL of the resulting solution to 100 mL with solution B and filter through a 0.45-µm filter (Millipore Millex is suitable). For solution (2) dilute 1 volume of solution (1) to 100 volumes with solution B and further dilute 1 volume to 2 volumes with the same mixture of solvents. Solution (3) contains 0.050% w/v of sulindac BPCRS (which has an assigned content of the E-isomer) in solution B. For solution (4) add 0.05 mL of a 3% w/v solution of potassium permanganate to 5 mL of solution (1) (generation of sulfoxide impurity). For solution (5) add a few granules of zinc, in fine powder to 5 mL of solution (1), add 0.2 mL of hydrochloric acid, mix and filter through a 0.45-µm filter (generation of desoxy impurity). For solution (6) add 0.3 mL of methanol to 5 mL of solution (1), add 0.2 mL of sulfuric acid and mix (generation of methyl ester). For solution (7) add 0.3 mL of absolute ethanol to 5 mL of solution (1), add 0.2 mL of sulfuric acid and mix (generation of ethyl ester).
The chromatographic procedure may be carried out using (a) a stainless steel column (15 cm × 4.6 mm) packed with octylsilyl silica gel for chromatography (5 µm) (Beckman Ultrasphere C8 is suitable), (b) as mobile phases A and B with a flow rate of 2 mL per minute the solutions described below and (c) a detection wavelength of 330 nm. Carry out a linear gradient elution using the following conditions.
Inject 20 µL of solution (3) and continue the chromatography for twice the retention time of the principal peak. The chromatogram obtained shows a principal peak corresponding to sulindac and a peak corresponding to the E-isomer with a retention time relative to sulindac of about 1.25.
From the chromatograms obtained with solution (1) and solution (3) calculate the content of E-isomer in the preparation being examined using the declared content of the E-isomer in sulindac BPCRS; the E-isomer content is not more than 0.5%. In the chromatogram obtained with solution (1), the area of any peaks corresponding to the desoxy impurity, the sulfoxide impurity, the ethyl ester impurity or the methyl ester impurity (identified by reference to the chromatograms obtained with solutions (4) to (7)) are not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.5%) and the sum of the areas of all the secondary peaks is not greater than twice the area of the principal peak in the chromatogram obtained with solution (2) (1%).
Assay
Weigh and powder 20 tablets. Shake a quantity of the powdered tablets containing 0.1 g of Sulindac with 80 mL of 0.1m methanolic hydrochloric acid for 10 minutes and dilute to 100 mL with the same solvent. Filter and dilute 3 mL of the filtrate to 200 mL with the same solvent. Measure the absorbance of the resulting solution at the maximum at 327 nm, Appendix II B, and calculate the content of C20H17FO3S taking 373 as the value of A(1%, 1 cm) at the maximum at 327 nm.
Storage
Sulindac Tablets should be protected from light.