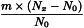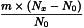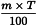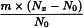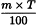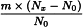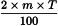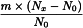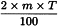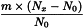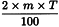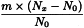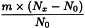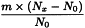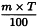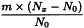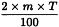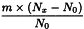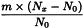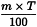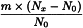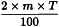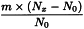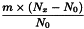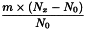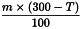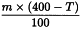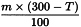Methods of Preparation of Homoeopathic Stocks and Potentisation
Homoeopathic stocks are prepared, using suitable methods, from raw materials that comply with the requirements of the monograph Homoeopathic preparations (1038). The methods described below, combined with established methods for potentisation, are examples of methods, but other methods described in an official national pharmacopoeia of a Member State may equally be used.
Where material of animal or human origin is to be used, particular reference is made to the requirements concerning the use of such raw material of zoological or human origin in the monograph Homoeopathic preparations (1038).
In the preparation of liquid dilutions, the ethanol of the concentration prescribed in the method may, if necessary, be replaced by ethanol (36 per cent V/V) or ethanol (18 per cent V/V).
When the individual monograph allows that the mother tincture be prepared from more than one plant species, the mother tincture can be prepared from the specified parts of an individual plant species or from any mixture thereof. If for the preparation of a mother tincture the loss on drying has to be determined, the herbal drug or mixture of herbal drug with ethanol has to be processed immediately after the value of the loss on drying has been determined.
Unless otherwise stated, mother tinctures are prepared by maceration lasting 10-30 days.
Maceration may be replaced by long maceration (maximum 60 days) or very long maceration (maximum 180 days), provided it is demonstrated that the quality of the resulting mother tincture is the same as that of the mother tincture prepared by maceration.
Unless otherwise stated in the individual monograph, the term ‘part(s)’ denotes ‘mass part(s)’. Unless otherwise stated in the method, the maximum temperature for the preparation is 25 °C.
1. mother tinctures and liquid potentisations
method 1.1. Hydroalcoholic mother tinctures prepared without heating
method 1.1.1 (Homöopathisches Arzneibuch (HAB) 1a: MOTHER TINCTURES AND LIQUID DILUTIONS)
Method 1.1.1 is used for fresh herbal drugs containing generally more than 70 per cent of expressed juice and no essential oil or resin or mucilage. Mother tinctures prepared according to Method 1.1.1 are mixtures of equal parts of expressed juices and ethanol (90 per cent V/V).
Express the comminuted herbal drug. Immediately mix the expressed juice with an equal mass of ethanol (90 per cent V/V). Allow to stand in a closed container for not less than 5 days, then filter.
Adjustment to any value specified in the individual monograph
Determine the percentage dry residue (2.8.16) or, where prescribed, the percentage assay content of the above-mentioned filtrate. Calculate the amount (A1), in kilograms, of ethanol (50 per cent V/V) required, using the following expression:
m | = | mass of filtrate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the filtrate. |
Mix the filtrate with the calculated amount of ethanol (50 per cent V/V). Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2.
The 1st‘centesimal’ dilution (C1) is made from:
The 2nd centesimal dilution (C2) is made from:
Subsequent centesimal dilutions are produced as stated for C2.
Method 1.1.2 (HAB 1b: MOTHER TINCTURES AND LIQUID DILUTIONS)
Method 1.1.2 is used where the latex of a herbal drug is to be processed.
Mother tinctures prepared according to Method 1.1.2 are mixtures of fresh plant latex with ethanol (36 per cent V/V). Mix the fresh latex with 2 parts by mass of ethanol (36 per cent V/V) and filter.
Adjustment to any value specified in the individual monograph
Determine the percentage dry residue (2.8.16) or, where prescribed, the percentage assay content of the above-mentioned filtrate. Calculate the amount (A1), in kilograms, of ethanol (36 per cent V/V) required, using the following expression:
m | = | mass of filtrate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the filtrate. |
Mix the filtrate with the calculated amount of ethanol (36 per cent V/V). Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2.
Method 1.1.3 (HAB 2a: MOTHER TINCTURES AND LIQUID DILUTIONS)
Method 1.1.3 is used for fresh herbal drugs containing generally less than 70 per cent of expressed juice and more than 60 per cent moisture (loss on drying) and no essential oil or resin.
Mother tinctures prepared according to Method 1.1.3 (ethanol content approximately 50 per cent V/V) are prepared by maceration as described below.
Comminute appropriately the herbal drug.
Take a sample and determine the loss on drying (2.2.32). Unless otherwise prescribed, determine the loss on drying on 2.00-5.00 g of the comminuted herbal drug and dry at 105 °C for 2 h.
To the comminuted herbal drug immediately add not less than half the mass of ethanol (90 per cent V/V) and allow to stand in well-closed containers.
Use the following expression to calculate the amount (A2), in kilograms, of ethanol (90 per cent V/V) required for the mass (m) of raw material, then subtract the amount of ethanol (90 per cent V/V) already added and add the difference to the mixture.
m | = | mass of raw material, in kilograms; |
T | = | percentage loss on drying of the sample. |
Allow to stand for not less than 10 days, swirling from time to time, then express the mixture and filter the resulting liquid.
Adjustment to any value specified in the individual monograph
Determine the percentage dry residue (2.8.16) or, where prescribed, the percentage assay content of the above-mentioned filtrate. Calculate the amount (A1), in kilograms, of ethanol (50 per cent V/V) required, using the following expression:
m | = | mass of filtrate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the filtrate. |
Mix the filtrate with the calculated amount of ethanol (50 per cent V/V). Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2.
The 1st‘centesimal’ dilution (C1) is made from:
The 2nd centesimal dilution (C2) is made from:
Subsequent centesimal dilutions are produced as stated for C2.
Method 1.1.4 (HAB 2b: MOTHER TINCTURES AND LIQUID DILUTIONS)
Method 1.1.4 is used for fresh herbal drugs containing generally less than 70 per cent of expressed juice and more than 60 per cent moisture (loss on drying) and no essential oil or resin.
Mother tinctures prepared according to Method 1.1.4 (ethanol content approximately 36 per cent V/V) are prepared by maceration as described below.
Comminute appropriately the herbal drug.
Take a sample and determine the loss on drying (2.2.32). Unless otherwise prescribed, determine the loss on drying on 2.00-5.00 g of the comminuted herbal drug and dry at 105 °C for 2 h.
To the comminuted herbal drug immediately add not less than half the mass of ethanol (70 per cent V/V) and allow to stand in well-closed containers.
Use the following expression to calculate the amount (A2), in kilograms, of ethanol (70 per cent V/V) required for the mass (m) of raw material, then subtract the amount of ethanol (70 per cent V/V) already added and add the difference to the mixture.
m | = | mass of raw material, in kilograms; |
T | = | percentage loss on drying of the sample. |
Allow to stand for not less than 10 days, swirling from time to time, then express the mixture and filter the resulting liquid.
Adjustment to any value specified in the individual monograph
Determine the percentage dry residue (2.8.16) or, where prescribed, the percentage assay content of the above-mentioned filtrate. Calculate the amount (A1), in kilograms, of ethanol (36 per cent V/V) required, using the following expression:
m | = | mass of filtrate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the filtrate. |
Mix the filtrate with the calculated amount of ethanol (36 per cent V/V). Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2.
Method 1.1.5 (HAB 3a: MOTHER TINCTURES AND LIQUID DILUTIONS)
Method 1.1.5 is used for fresh herbal drugs containing essential oil or resin, or generally less than 60 per cent moisture.
Mother tinctures prepared according to Method 1.1.5 (ethanol content approximately 65 per cent V/V) are prepared by maceration as described below.
Comminute appropriately the herbal drug.
Determine the water content (2.2.13) or loss on drying (2.2.32). Unless otherwise prescribed, determine the loss on drying on 2.00-5.00 g of the comminuted herbal drug and dry at 105 °C for 2 h.
To the comminuted herbal drug immediately add not less than half the mass of ethanol (90 per cent V/V) and allow to stand in well-closed containers.
Use the following expression to calculate the amount (A3), in kilograms, of ethanol (90 per cent V/V) required for the mass (m) of raw material, then subtract the amount of ethanol (90 per cent V/V) already added and add the difference to the mixture.
m | = | mass of raw material, in kilograms; |
T | = | percentage loss on drying of the sample. |
Allow to stand for not less than 10 days, swirling from time to time, then express the mixture and filter the resulting liquid.
Adjustment to any value specified in the individual monograph
Determine the percentage dry residue (2.8.16) or, where prescribed, the percentage assay content of the above-mentioned filtrate. Calculate the amount (A1), in kilograms, of ethanol (70 per cent V/V) required, using the following expression:
m | = | mass of filtrate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the filtrate. |
Mix the filtrate with the calculated amount of ethanol (70 per cent V/V). Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2. Use ethanol (50 per cent V/V) for dilutions from D4 onwards.
The 1st‘centesimal’ dilution (C1) is made from:
The 2nd centesimal dilution (C2) is made from:
Subsequent centesimal dilutions are produced as stated for C2.
Method 1.1.6 (HAB 3b: MOTHER TINCTURES AND LIQUID DILUTIONS)
Method 1.1.6 is used for fresh herbal drugs containing essential oils or resins or generally less than 60 per cent moisture.
Mother tinctures prepared according to Method 1.1.6 (ethanol content approximately 57 per cent V/V) are prepared by maceration as described below.
Comminute appropriately the herbal drug.
Determine the water content (2.2.13) or loss on drying (2.2.32). Unless otherwise prescribed, determine the loss on drying on 2.00-5.00 g of the comminuted herbal drug and dry at 105 °C for 2 h.
To the comminuted herbal drug immediately add not less than half the mass of ethanol (80 per cent V/V) and allow to stand in well-closed containers.
Use the following expression to calculate the amount (A3), in kilograms, of ethanol (80 per cent V/V) required for the mass (m) of raw material, then subtract the amount of ethanol (80 per cent V/V) already added and add the difference to the mixture.
m | = | mass of raw material, in kilograms; |
T | = | percentage loss on drying of the sample. |
Allow to stand for not less than 10 days, swirling from time to time, then express the mixture and filter the resulting liquid.
Adjustment to any value specified in the individual monograph
Determine the percentage dry residue (2.8.16) or, where prescribed, the percentage assay content of the above-mentioned filtrate. Calculate the amount (A1), in kilograms, of ethanol (50 per cent V/V) required, using the following expression:
m | = | mass of filtrate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the filtrate. |
Mix the filtrate with the calculated amount of ethanol (50 per cent V/V). Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
The 3rd decimal dilution (D3) is made from:
Subsequent decimal dilutions are produced as stated for D3.
Method 1.1.7 (HAB 3c: MOTHER TINCTURES AND LIQUID DILUTIONS)
Method 1.1.7 is used for fresh herbal drugs containing generally less than 60 per cent moisture (loss on drying).
Mother tinctures prepared according to Method 1.1.7 (ethanol content approximately 35 per cent V/V) are prepared by maceration as described below.
Comminute appropriately the herbal drug.
Take a sample and determine the loss on drying (2.2.32). Unless otherwise prescribed, determine the loss on drying on 2.00-5.00 g of the comminuted herbal drug and dry at 105 °C for 2 h.
To the comminuted herbal drug immediately add not less than half the mass of ethanol (50 per cent V/V) and allow to stand in well-closed containers.
Use the following expression to calculate the amount (A3), in kilograms, of ethanol (50 per cent V/V) required for the mass (m) of raw material, then subtract the amount of ethanol (50 per cent V/V) already added and add the difference to the mixture.
m | = | mass of raw material, in kilograms; |
T | = | percentage loss on drying of the sample. |
Allow to stand for not less than 10 days, swirling from time to time, then express the mixture and filter the resulting liquid.
Adjustment to any value specified in the individual monograph
Determine the percentage dry residue (2.8.16) or, where prescribed, the percentage assay content of the above-mentioned filtrate. Calculate the amount (A1), in kilograms, of ethanol (36 per cent V/V) required, using the following expression:
m | = | mass of filtrate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the filtrate. |
Mix the filtrate with the calculated amount of ethanol (36 per cent V/V). Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2.
Method 1.1.8 (HAB 4a: MOTHER TINCTURES AND LIQUID DILUTIONS)
Method 1.1.8 is generally used for dried herbal drugs.
Mother tinctures prepared according to Method 1.1.8 are prepared by maceration or percolation as described below, using 1 part of dried herbal drug and 10 parts of ethanol of the appropriate concentration (anhydrous, 96 per cent V/V, 90 per cent V/V, 80 per cent V/V, 70 per cent V/V, 50 per cent V/V, 36 per cent V/V, 18 per cent V/V), unless otherwise prescribed in the individual monograph.
Production by maceration Unless otherwise prescribed, comminute the herbal drug, mix thoroughly with ethanol of the appropriate concentration and allow to stand in a closed container for an appropriate time. Separate the residue from the ethanol and, if necessary, press out. In the latter case, combine the 2 liquids obtained.
Production by percolation If necessary, comminute the herbal drug. Mix thoroughly with a portion of ethanol of the appropriate concentration and allow to stand for an appropriate time. Transfer to a percolator and allow the percolate to flow slowly, at room temperature, making sure that the herbal drug to be extracted is always covered with the remaining ethanol. The residue may be pressed out and the expressed liquid combined with the percolate.
If adjustment to a given concentration is necessary, calculate the amount (A1), in kilograms, of ethanol of the appropriate concentration required to obtain the concentration specified or used for production, using the following expression:
m | = | mass of percolate or macerate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the percolate or macerate. |
Mix the macerate or percolate with the calculated amount of ethanol of the appropriate concentration. Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The mother tincture corresponds to the 1st‘decimal’ dilution (Ø = D1).
The 2nd decimal dilution (D2) is made from:
The 3rd decimal dilution (D3) is made from:
Unless a different ethanol concentration is specified, use ethanol (50 per cent V/V) for subsequent decimal dilutions from D4 onwards and proceed as stated for D3.
The 1st‘centesimal’ dilution (C1) is made from:
The 2nd centesimal dilution (C2) is made from:
Subsequent centesimal dilutions are produced as stated for C2.
Method 1.1.9 (HAB 4b: MOTHER TINCTURES AND LIQUID DILUTIONS)
Method 1.1.9 is generally used for animal matter.
Mother tinctures prepared according to Method 1.1.9 are prepared by maceration or percolation as described below, using 1 part of animal matter and 10 parts of ethanol of the appropriate concentration (anhydrous, 96 per cent V/V, 90 per cent V/V, 80 per cent V/V, 70 per cent V/V, 50 per cent V/V, 36 per cent V/V, 18 per cent V/V), unless otherwise prescribed in the individual monograph.
Production by maceration Unless otherwise prescribed, comminute the animal matter, mix thoroughly with ethanol of the appropriate concentration and allow to stand in a closed container for an appropriate time. Separate the residue from the ethanol and, if necessary, press out. In the latter case, combine the 2 liquids obtained.
Production by percolation If necessary, comminute the animal matter. Mix thoroughly with a portion of ethanol of the appropriate concentration and allow to stand for an appropriate time. Transfer to a percolator and allow the percolate to flow slowly at room temperature, making sure that the animal matter to be extracted is always covered with the remaining ethanol. The residue may be pressed out and the expressed liquid combined with the percolate.
If adjustment to a given concentration is necessary, calculate the amount (A1), in kilograms, of ethanol of the appropriate concentration required to obtain the concentration specified or used for production, using the following expression:
m | = | mass of percolate or macerate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the percolate or macerate. |
Mix the macerate or percolate with the calculated amount of ethanol of the appropriate concentration. Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The mother tincture corresponds to the 1st‘decimal’ dilution (Ø = D1).
The 2nd decimal dilution (D2) is made from:
The 3rd decimal dilution (D3) is made from:
Unless a different ethanol concentration is specified, use ethanol (50 per cent V/V) for subsequent decimal dilutions from D4 onwards and proceed as stated for D3.
The 1st‘centesimal’ dilution (C1) is made from:
The 2nd centesimal dilution (C2) is made from:
Subsequent centesimal dilutions are produced as stated for C2.
Method 1.1.10 (french pharmacopoeia)
Method 1.1.10 is generally used for herbal drugs. The state of the herbal drug, fresh or dried, is specified in the individual monograph.
Mother tinctures prepared according to Method 1.1.10 are prepared by maceration.
Comminute appropriately the herbal drug. Take a sample and determine the loss on drying at 105 °C for 2 h (2.2.32) or the water content (2.2.13). Taking this value into account, calculate and add to the herbal drug the quantities of ethanol of the appropriate concentration required to produce, unless otherwise prescribed, a 1 in 10 mother tincture (1:10 mother tincture) with a suitable ethanol content. Allow to macerate for at least 10 days, with sufficient shaking.
Separate the residue from the ethanol and strain under pressure if necessary. Allow the combined liquids to stand for 48 h and filter. For mother tinctures with a required assay content, adjustment may be carried out, if necessary, by adding ethanol of the same concentration as used for the preparation of the tincture.
Potentisation
The 1st decimal dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2, using ethanol of the appropriate concentration.
The 1st centesimal dilution (C1) is made from:
The 2nd centesimal dilution (C2) is made from:
Subsequent centesimal dilutions are produced as stated for C2, using ethanol of the appropriate concentration.
Method 1.1.11 (french pharmacopoeia)
Method 1.1.11 is generally used for animal matter.
Mother tinctures prepared according to Method 1.1.11 are prepared by maceration.
The mass ratio of raw material to mother tincture is usually 1 to 20. To the raw material, appropriately comminuted, add the quantity of ethanol of the appropriate concentration required to produce a 1 in 20 mother tincture. Allow to macerate for at least 10 days, with sufficient shaking. Decant and filter. Allow to stand for 48 h and filter again. For mother tinctures with a required assay content, adjustment may be carried out, if necessary, by adding ethanol of the same concentration as used for the preparation of the tincture.
Potentisation
The 1st decimal dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2, using ethanol of the appropriate concentration.
The 1st centesimal dilution (C1) is made from:
The 2nd centesimal dilution (C2) is made from:
Subsequent centesimal dilutions are produced as stated for C2, using ethanol of the appropriate concentration.
METHOD 1.2. hydroalcoholic MOTHER TINCTURES prepared with heating
METHODS 1.2.1, 1.2.2. ETHANOLIC DIGESTIONS (HAB 18a, 18b: HEAT-TREATED MOTHER TINCTURES)
Methods 1.2.1 and 1.2.2 are used for fresh herbal drugs containing generally less than 70 per cent of expressed juice and more than 60 per cent moisture (loss on drying) and no essential oil or resin.
Mother tinctures prepared according to Methods 1.2.1 (ethanol content approximately 50 per cent V/V) and 1.2.2 (ethanol content approximately 36 per cent V/V) are ethanolic digestions prepared by heat treatment and additional maceration as described below.
Comminute appropriately the herbal drug.
Take a sample and determine the loss on drying (2.2.32). Unless otherwise prescribed, determine the loss on drying on 2.00-5.00 g of the comminuted herbal drug and dry at 105 °C for 2 h.
To the comminuted herbal drug immediately add not less than half the mass of ethanol of the concentration prescribed below and allow to stand in well-closed containers:
Use the following expression to calculate the amount (A2), in kilograms, of ethanol of the appropriate concentration required for the mass (m) of raw material, then subtract the amount of ethanol of the appropriate concentration already added and add the difference to the mixture.
m | = | mass of raw material, in kilograms; |
T | = | percentage loss on drying of the sample. |
Warm the mixture containing the total amount of ethanol of the appropriate concentration to 37 °C in a covered container and maintain at this temperature for 1 h, swirling from time to time. Cool, allow to stand for not less than 10 days, swirling from time to time, then express the mixture and filter the resulting liquid.
Adjustment to any value as specified in the individual monograph
Determine the percentage dry residue (2.8.16) or, where prescribed, the percentage assay content of the above-mentioned filtrate. Calculate the amount (A1), in kilograms, of ethanol of the appropriate concentration required, using the following expression:
m | = | mass of filtrate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the filtrate. |
Mix the filtrate with the required amount of ethanol of the concentration prescribed below:
Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
The 3rd decimal dilution (D3) is made from:
Subsequent decimal dilutions are produced as stated for D3.
METHODS 1.2.3, 1.2.4, 1.2.5. ETHANOLIC DIGESTIONS (HAB 18c, 18d, 18e: HEAT-TREATED MOTHER TINCTURES)
Methods 1.2.3, 1.2.4 and 1.2.5 are used for fresh herbal drugs containing essential oil or resin or generally less than 60 per cent moisture.
Mother tinctures prepared according to Methods 1.2.3 (ethanol content approximately 65 per cent V/V), 1.2.4 (ethanol content approximately 57 per cent V/V) and 1.2.5 (ethanol content approximately 35 per cent V/V) are ethanolic digestions prepared by heat treatment and additional maceration as described below.
Comminute appropriately the herbal drug.
Determine the water content (2.2.13) or loss on drying (2.2.32). Unless otherwise prescribed, determine the loss on drying on 2.00-5.00 g of the comminuted herbal drug and dry at 105 °C for 2 h.
To the comminuted herbal drug immediately add not less than half the mass of ethanol of the concentration prescribed below and allow to stand in well-closed containers:
Use the following expression to calculate the amount (A3), in kilograms, of ethanol of the appropriate concentration required for the mass (m) of raw material, then subtract the amount of ethanol of the appropriate concentration already added and add the difference to the mixture.
m | = | mass of raw material, in kilograms; |
T | = | percentage loss on drying of the sample. |
Warm the mixture containing the total amount of ethanol of the appropriate concentration to 37 °C in a covered container and maintain at this temperature for 1 h, swirling from time to time. Cool, allow to stand for not less than 10 days, swirling from time to time, then express the mixture and filter the resulting liquid.
Adjustment to any value as specified in the individual monograph
Determine the percentage dry residue (2.8.16) or, where prescribed, the percentage assay content of the above-mentioned filtrate. Calculate the amount (A1), in kilograms, of ethanol of the appropriate concentration required, using the following expression:
m | = | mass of filtrate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the filtrate. |
Mix the filtrate with the required amount of ethanol of the concentration prescribed below:
Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced accordingly. In this process the ethanol concentration is reduced with each step, according to the sequence 70 per cent V/V - 50 per cent V/V - 36 per cent V/V - 18 per cent V/V.
METHOD 1.2.6. ETHANOLIC DIGESTIONS (HAB 18f: HEAT-TREATED MOTHER TINCTURES)
Method 1.2.6 is used for dried herbal drugs.
Mother tinctures prepared according to Method 1.2.6 are ethanolic digestions prepared by heat treatment and additional maceration as described below, using 1 part of dried herbal drug and 10 parts of ethanol of the appropriate concentration (96 per cent V/V, 90 per cent V/V, 80 per cent V/V, 70 per cent V/V, 50 per cent V/V, 36 per cent V/V, 18 per cent V/V), unless otherwise prescribed in the individual monograph.
Unless otherwise prescribed, comminute appropriately the herbal drug, mix thoroughly with the total amount of ethanol of the appropriate concentration, warm the mixture to 37 °C in a covered container and maintain at this temperature for 1 h, swirling from time to time. Cool, then allow to stand in a closed container for an appropriate time. After sedimentation, decant the supernatant and, if necessary, press out. In the latter case, combine the 2 liquids obtained. Filter the resulting liquid.
Adjustment to any value as specified in the individual monograph
Determine the percentage dry residue (2.8.16) or, where prescribed, the percentage assay content of the above-mentioned filtrate. Calculate the amount (A1), in kilograms, of ethanol of the appropriate concentration required to obtain the concentration specified or used for production, using the following expression:
m | = | mass of the filtrate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the filtrate. |
Mix the filtrate with the calculated amount of ethanol of the appropriate concentration. Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The mother tincture corresponds to the 1st‘decimal’ dilution (Ø = D1).
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced accordingly. In this process the ethanol concentration is reduced with each step, according to the sequence 96 per cent V/V - 90 per cent V/V - 80 per cent V/V - 70 per cent V/V - 50 per cent V/V - 36 per cent V/V - 18 per cent V/V.
METHODS 1.2.7, 1.2.8. ETHANOLIC DECOCTIONS (HAB 19a, 19b: HEAT-TREATED MOTHER TINCTURES)
Methods 1.2.7 and 1.2.8 are used for fresh herbal drugs containing generally less than 70 per cent of expressed juice and more than 60 per cent moisture (loss on drying) and no essential oil or resin.
Mother tinctures prepared according to Methods 1.2.7 (ethanol content approximately 50 per cent V/V) and 1.2.8 (ethanol content approximately 36 per cent V/V) are ethanolic decoctions prepared by heat treatment and additional maceration as described below.
Comminute appropriately the herbal drug.
Take a sample and determine the loss on drying (2.2.32). Unless otherwise prescribed, determine the loss on drying on 2.00-5.00 g of the comminuted herbal drug and dry at 105 °C for 2 h.
To the comminuted herbal drug immediately add not less than half the mass of ethanol of the concentration prescribed below and allow to stand in well-closed containers:
Use the following expression to calculate the amount (A2), in kilograms, of ethanol of the appropriate concentration required for the mass of raw material, then subtract the amount of ethanol of the appropriate concentration already added and add the difference to the mixture.
m | = | mass of raw material, in kilograms; |
T | = | percentage loss on drying of the sample. |
Heat the mixture containing the total amount of ethanol of the appropriate concentration to boiling under reflux, and maintain for 30 min, unless otherwise specified in the individual monograph. Cool or allow to cool, allow to stand in a closed container for 12-36 h, then express the mixture and filter the resulting liquid.
Adjustment to any value as specified in the individual monograph
Determine the percentage dry residue (2.8.16) or, where prescribed, the percentage assay content of the above-mentioned filtrate. Calculate the amount (A1), in kilograms, of ethanol of the appropriate concentration required, using the following expression:
m | = | mass of filtrate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the filtrate. |
Mix the filtrate with the required amount of ethanol of the concentration prescribed below:
Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
The 3rd decimal dilution (D3) is made from:
Subsequent decimal dilutions are produced as stated for D3.
METHODS 1.2.9, 1.2.10, 1.2.11. ETHANOLIC DECOCTIONS (HAB 19c, 19d, 19e: HEAT-TREATED MOTHER TINCTURES)
Methods 1.2.9, 1.2.10 and 1.2.11 are used for fresh herbal drugs containing essential oil or resin or generally less than 65 per cent moisture.
Mother tinctures prepared according to Methods 1.2.9 (ethanol content approximately 65 per cent V/V), 1.2.10 (ethanol content approximately 57 per cent V/V) and 1.2.11 (ethanol content approximately 35 per cent V/V) are ethanolic decoctions prepared by heat treatment and additional maceration as described below.
Comminute appropriately the herbal drug.
Determine the water content (2.2.13) or loss on drying (2.2.32). Unless otherwise prescribed, determine the loss on drying on 2.00-5.00 g of the comminuted herbal drug and dry at 105 °C for 2 h.
To the comminuted herbal drug immediately add not less than half the mass of ethanol of the concentration prescribed below and allow to stand in well-closed containers:
Use the following expression to calculate the amount (A3), in kilograms, of ethanol of the appropriate concentration required for the mass of raw material, then subtract the amount of ethanol of the appropriate concentration already added and add the difference to the mixture.
m | = | mass of raw material, in kilograms; |
T | = | percentage loss on drying of the sample. |
Heat the mixture containing the total amount of ethanol of the appropriate concentration to boiling under reflux, and maintain for 30 min, unless otherwise specified in the individual monograph. Cool or allow to cool, then allow to stand in a closed container for 12-36 h, express the mixture and filter the resulting liquid.
Adjustment to any value as specified in the individual monograph
Determine the percentage dry residue (2.8.16) or, where prescribed, the percentage assay content of the above-mentioned filtrate. Calculate the amount (A1), in kilograms, of ethanol of the appropriate concentration required, using the following expression:
m | = | mass of filtrate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the filtrate. |
Mix the filtrate with the required amount of ethanol of the concentration prescribed below:
Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced accordingly. In this process the ethanol concentration is reduced with each step, according to the sequence 70 per cent V/V - 50 per cent V/V - 36 per cent V/V - 18 per cent V/V.
METHOD 1.2.12. ETHANOLIC DECOCTIONS (HAB 19f: HEAT-TREATED MOTHER TINCTURES)
Method 1.2.12 is used for dried herbal drugs.
Mother tinctures prepared according to Method 1.2.12 are ethanolic decoctions prepared by heat treatment and additional maceration as described below, using 1 part of dried herbal drug and 10 parts of ethanol of the appropriate concentration (96 per cent V/V, 90 per cent V/V, 80 per cent V/V, 70 per cent V/V, 50 per cent V/V, 36 per cent V/V, 18 per cent V/V), unless otherwise prescribed in the individual monograph.
Unless otherwise prescribed, comminute appropriately the herbal drug, mix thoroughly with the total amount of ethanol of the appropriate concentration, heat to boiling under reflux and maintain for 30 min. Cool or allow to cool, then allow to stand in a closed container for 12-36 h. After sedimentation, decant the supernatant and, if necessary, press out. In the latter case, combine the 2 liquids obtained. Filter the resulting liquid.
Adjustment to any value as specified in the individual monograph
Determine the percentage dry residue (2.8.16) or, where prescribed, the percentage assay content of the above-mentioned filtrate. Calculate the amount (A1), in kilograms, of ethanol of the appropriate concentration required to obtain the concentration specified or used for production, using the following expression:
m | = | mass of filtrate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the filtrate. |
Mix the filtrate with the required amount of ethanol of the appropriate concentration. Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The mother tincture corresponds to the 1st‘decimal’ dilution (Ø = D1).
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced accordingly. In this process the ethanol concentration is reduced with each step, according to the sequence 96 per cent V/V - 90 per cent V/V - 80 per cent V/V - 70 per cent V/V - 50 per cent V/V - 36 per cent V/V - 18 per cent V/V.
METHOD 1.2.13. ETHANOLIC INFUSIONS (HAB 20: HEAT-TREATED MOTHER TINCTURES)
Method 1.2.13 is used for dried herbal drugs.
Mother tinctures prepared according to Method 1.2.13 are ethanolic infusions prepared by heat treatment and additional maceration as described below using 1 part of dried herbal drug and 10 parts of ethanol of the appropriate concentration (90 per cent V/V, 80 per cent V/V, 70 per cent V/V, 50 per cent V/V, 36 per cent V/V, 18 per cent V/V), unless otherwise prescribed in the individual monograph. The quantities of ethanol (96 per cent V/V) and purified water required to achieve the specified ethanol concentration are added separately as described below.
Unless otherwise prescribed, comminute appropriately the herbal drug, mix thoroughly with the total amount of ethanol (96 per cent V/V), cover and allow to stand for 15 min. Add the purified water (heated to boiling) and heat the mixture to boiling under reflux for 5 min. Cool, or allow to cool, then allow to stand in a closed container for 12-36 h. After sedimentation, decant the supernatant and, if necessary, press out. In the latter case, combine the 2 liquids obtained. Filter the resulting liquid.
Adjustment to any value as specified in the individual monograph
Determine the percentage dry residue (2.8.16) or, where prescribed, the percentage assay content of the above-mentioned filtrate. Calculate the amount (A1), in kilograms, of ethanol of the appropriate concentration required to obtain the concentration specified or used for production, using the following expression:
m | = | mass of filtrate, in kilograms; |
N0 | = | percentage dry residue or percentage assay content as required in the individual monograph; |
Nx | = | percentage dry residue or percentage assay content of the filtrate. |
Mix the filtrate with the required amount of ethanol of the appropriate concentration. Allow to stand for not less than 5 days, then filter if necessary.
Potentisation
The mother tincture corresponds to the 1st‘decimal’ dilution (Ø = D1).
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced accordingly. In this process the ethanol concentration is reduced with each step, according to the sequence 90 per cent V/V - 80 per cent V/V - 70 per cent V/V - 50 per cent V/V - 36 per cent V/V - 18 per cent V/V.
method 1.3. Aqueous mother tinctures prepared without heating
Method 1.3.1. AQUEOUS MACERATES (HAB 49: AQUEOUS MOTHER TINCTURES)
Method 1.3.1 is used for fresh herbal drugs.
Mother tinctures prepared according to Method 1.3.1 are aqueous macerates prepared by short maceration with water as described below. This method is used solely in the manufacture of injections and eye preparations.
Comminute appropriately the herbal drug.
Take a sample and determine the loss on drying (2.2.32). Unless otherwise prescribed, determine the loss on drying on 2.00-5.00 g of the comminuted herbal drug and dry at 105 °C for 2 h.
Use the following expression to calculate the amount, in kilograms, of water required for the mass of raw material:
m | = | mass of raw material, in kilograms; |
T | = | percentage loss on drying of the sample. |
Add the comminuted herbal drug to the calculated amount of water. Allow to stand for 2 h, then express the mixture and filter the resulting liquid. The mother tincture is used immediately, unless otherwise justified.
Potentisation
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2.
Method 1.4. Aqueous mother tinctures Prepared with heating
METHOD 1.4.1. AQUEOUS DIGESTIONS (HAB 24b: HEAT-TREATED aqueous MOTHER TINCTURES)
Method 1.4.1 is used for fresh herbal drugs.
Mother tinctures prepared according to Method 1.4.1 are aqueous digestions prepared by heat treatment with water as described below. This method is solely used in the preparation of injections, eye preparations and coated homoeopathic pillules.
Comminute appropriately the herbal drug.
Take a sample and determine the loss on drying (2.2.32). Unless otherwise prescribed, determine the loss on drying on 2.00-5.00 g of the comminuted herbal drug and dry at 105 °C for 2 h.
Use the following expression to calculate the amount, in kilograms, of water required for the mass of raw material:
m | = | mass of raw material, in kilograms; |
T | = | percentage loss on drying of the sample. |
Heat the mixture containing the total amount of water in a covered container to 37 °C and maintain at this temperature for 1 h, swirling from time to time. Express the mixture and filter the resulting liquid. The mother tincture is used immediately, unless otherwise justified.
Potentisation
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2.
METHOD 1.4.2. AQUEOUS DECOCTIONS (HAB 23b: HEAT-TREATED aqueous MOTHER TINCTURES)
Method 1.4.2 is used for fresh herbal drugs.
Mother tinctures prepared according to Method 1.4.2 are aqueous decoctions prepared by heat treatment with water as described below. This method is solely used in the preparation of injections, eye preparations and coated homoeopathic pillules.
Comminute appropriately the herbal drug.
Take a sample and determine the loss on drying (2.2.32). Unless otherwise prescribed, determine the loss on drying on 2.00-5.00 g of the comminuted herbal drug and dry at 105 °C for 2 h.
Use the following expression to calculate the amount, in kilograms, of water required for the mass of raw material:
m | = | mass of raw material, in kilograms; |
T | = | percentage loss on drying of the sample. |
Heat the calculated amount of water to above 90 °C and add the comminuted herbal drug. Maintain the mixture at this temperature under reflux for 30 min, then express the mixture and filter the resulting liquid. The mother tincture is used immediately, unless otherwise justified.
Potentisation
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2.
METHOD 1.4.3. AQUEOUS DECOCTIONS (HAB 23a: HEAT-TREATED aqueous MOTHER TINCTURES)
Method 1.4.3 is used for dried herbal drugs.
Mother tinctures prepared according to Method 1.4.3 are aqueous decoctions prepared by heat treatment with water as described below. This method is solely used in the preparation of injections, eye preparations and coated homoeopathic pillules.
Comminute appropriately the herbal drug.
Mix 1 part of the comminuted dried herbal drug with 10 parts of boiling water and boil under reflux for 30 min, unless otherwise specified in the individual monograph. Filter while hot. If gentle pressure applied to the residue does not achieve a final mass of mother tincture equal to 10 parts, pour a sufficient amount of boiling water over the residue and express gently. Use the resulting extract to make the mother tincture up to 10 parts. Filter the combined liquid. The filtrate is the mother tincture. The mother tincture is used immediately, unless otherwise justified.
For starch-containing material process 1 part of herbal drug with 100 parts of water. In that case the mother tincture corresponds to the 2nd decimal dilution (Ø = D2).
Potentisation
The mother tincture corresponds to the 1st‘decimal’ dilution (Ø = D1).
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2.
METHOD 1.4.4. AQUEOUS INFUSIONS (HAB 24a: HEAT-TREATED aqueous MOTHER TINCTURES)
Method 1.4.4 is used for dried herbal drugs.
Mother tinctures prepared according to Method 1.4.4 are aqueous infusions prepared by heat treatment with water and additional maceration as described below. This method is solely used in the preparation of injections, eye preparations and coated homoeopathic pillules.
Comminute appropriately the herbal drug.
1 part of dried herbal drug is extracted with 10 parts of water.
Grind 1 part of the comminuted herbal drug in a mortar thoroughly with 3-5 parts of water, allow to stand for 15 min, then add the remainder of the water, which has been heated to boiling point. Place the mixture in a water-bath and maintain at a temperature above 90 °C for 5 min, swirling from time to time. Cover, allow to cool, then separate the residue from the liquid. If gentle pressure applied to the residue does not achieve a final mass of mother tincture equal to 10 parts, pour a sufficient amount of cold water over the residue and express gently. Use the resulting extract to make the mother tincture up to 10 parts. Filter the combined liquid. The filtrate is the mother tincture. The mother tincture is used immediately, unless otherwise justified.
Potentisation
The mother tincture corresponds to the 1st‘decimal’ dilution (Ø = D1).
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2.
2. GLYCEROL MACERATES
METHOD 2.1
Method 2.1 is used for maceration of raw materials of animal or herbal origin in glycerol (85 per cent) or glycerol/ethanol mixtures of appropriate concentration. Pathological material is excluded.
The raw materials are finely minced before use, where appropriate.
METHODS 2.1.1, 2.1.2 (HAB 42a AND 42b: MOTHER TINCTURES AND LIQUID DILUTIONS THEREOF)
Raw materials of animal origin - freshly killed animals or parts thereof - are used. Animals are processed immediately after being killed.
Maceration
Disperse 1 part of finely minced animal material in:
Allow to macerate for at least 2 h, then succuss. Filter when necessary.
Where justified, 1 part of glycerol (85 per cent) may be added to 1 part of animal material before mincing. Where very small amounts of animal material are used, the dilution may be prepared by dispersing 1 part of finely minced animal material in 99 parts of glycerol (85 per cent) (C1 or ‘D2’ if to be used for further decimal dilutions).
Potentisation
Method 2.1.1
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2 but with ethanol (18 per cent V/V) as the vehicle.
The 2nd centesimal dilution (C2) is made from:
Subsequent centesimal dilutions are produced as stated for C2.
Method 2.1.2
The 1st‘decimal’ dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2.
METHOD 2.1.3 (french pharmacopoeia)
Raw materials of herbal or animal origin are used.
Maceration
Comminute the raw material appropriately. Take a sample and determine the loss on drying at 105 °C for 2 h (2.2.32) or the water content (2.2.13). Taking this value into account, calculate and add to the raw material the quantity of the ethanol/glycerol mixture of the appropriate concentration to produce, unless otherwise prescribed, a 1 in 20 glycerol macerate. Allow to macerate for at least 21 days, with sufficient shaking. Decant and strain under pressure if necessary. Allow the combined liquids to stand for 48 h and filter.
Potentisation
The 1st decimal dilution (D1) is made from:
The 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2 or using another appropriate vehicle.
The 1st centesimal dilution (C1) is made from:
The 2nd centesimal dilution (C2) is made from:
Subsequent centesimal dilutions are produced as stated for C2 or using another appropriate vehicle.
METHOD 2.2
METHODS 2.2.1, 2.2.2, 2.2.3, 2.2.4 (HAB 41a, 41b, 41c AND 41d: GLYCEROL MOTHER TINCTURES AND LIQUID DILUTIONS THEREOF)
Method 2.2 is used for maceration of raw materials of animal origin in a glycerol solution containing sodium chloride. Pathological material is excluded.
Raw materials from freshly killed animals, parts or secretions thereof are used in Methods 2.2.1, 2.2.2 and 2.2.3. Lower animals are killed with carbon dioxide in a covered vessel. All animals are processed immediately after being killed.
Blood components from live horses are used in method 2.2.4.
Sample collection and/or pre-treatment
The raw materials used in Methods 2.2.1, 2.2.2 and 2.2.3 are finely minced before use, where appropriate.
The blood used in Method 2.2.4 is collected by a veterinarian. Blood obtained from animals killed by bleeding must not be used. Take 200 mL of this blood and add 15 IU of heparin sodium and 0.625 mL of a 9 g/kg solution of sodium chloride per millilitre. Separate the blood components by fractional centrifugation and resuspend each individual cell sediment in 1.1 mL of a 9 g/kg solution of sodium chloride. These cell suspensions are processed into the glycerol macerate.
Maceration
Mix 1 part of finely minced animal material, secretions or blood cell suspensions, according to the method used, with 5 parts of a sodium chloride solution of the appropriate concentration (see Table 2371.-1) and 95 parts of glycerol. Allow to stand protected from light for at least 7 days, then decant. If necessary for Methods 2.2.1, 2.2.2 and 2.2.3, centrifuge before decanting, then filter the supernatant if necessary. The decanted liquid or the filtrate respectively is the glycerol macerate.
Any sediment present must be resuspended before processing the glycerol macerate.
Vehicle
0.2 parts of sodium hydrogen carbonate and 8.8 parts of sodium chloride in 991 parts of water for injections or purified water as appropriate.
Potentisation
The glycerol macerate corresponds to the 2nd decimal dilution (‘D2’) or the 1st centesimal dilution (C1).
The 3rd decimal dilution (D3) is made from:
Subsequent decimal dilutions are produced as stated for D3.
Where appropriate, the 4th decimal dilution (D4) is made from 1 part of the 3rd decimal dilution, 5.6 parts of the vehicle and 3.4 parts of water for injections.
The 2nd centesimal dilution (C2) is made from:
Subsequent centesimal dilutions are produced as stated for C2.
3. LIQUID DILUTIONS
METHOD 3.1
Methods 3.1.1, 3.1.2 and 3.1.3 are used for dissolution of any suitable inorganic or organic starting material, for example minerals or venoms.
Unless otherwise specified, dissolve 1 part of the starting material in 9 parts (D1) or 99 parts (C1) of the liquid vehicle and succuss.
Where justified and authorised, in case of insufficient solubility of the starting material in the specified vehicle, directly produce the first possible dilution. For example, if the starting material is slightly soluble, dissolve 1 part of the starting material in 99 parts of the vehicle (C1 or ‘D2’ if to be used for further decimal dilutions).
METHODS 3.1.1, 3.1.2 (HAB 5a, 5b: SOLUTIONS, AQUEOUS SOLUTIONS)
Vehicles
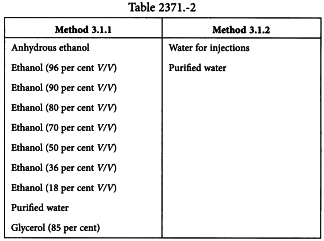
The vehicles in Table 2371.-2 may be used.
For Method 3.1.1, if ethanol (18 per cent V/V) is used, the starting material may be dissolved in 7.58 parts of purified water and the ethanol concentration adjusted by adding 1.42 parts of ethanol (96 per cent V/V) to the solution, for decimal dilutions. For centesimal dilutions, use 83.4 parts of purified water for 15.6 parts of ethanol (96 per cent V/V).
For Method 3.1.2, if the starting material is not stable and/or soluble in water, glycerol (85 per cent) may be added at a concentration of not more than 35 per cent of the vehicle, for potentisation up to D4.
Potentisation
Unless otherwise specified, the 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2.
Unless otherwise specified, the 2nd centesimal dilution (C2) is made from:
Subsequent centesimal dilutions are produced as stated for C2.
Additives
For Method 3.1.1, if a reaction such as precipitation is observed in the final dilution, the following additives may be used to enhance stability and/or solubility, unless otherwise specified:
Where solutions or dilutions have been pH-adjusted, they must not be potentised further.
METHOD 3.1.3
Vehicles
Suitable vehicles, for example, ethanol of an appropriate concentration, glycerol or purified water may be used alone or combined.
Potentisation
Unless otherwise specified, the 2nd decimal dilution (D2) is made from:
Subsequent decimal dilutions are produced as stated for D2.
Unless otherwise specified, the 2nd centesimal dilution (C2) is made from:
Subsequent centesimal dilutions are produced as stated for C2.
METHOD 3.2
Method 3.2 is generally used to produce liquid dilutions of triturations of substances that for the most part are sparingly soluble to practically insoluble.
methods 3.2.1, 3.2.2 (HAB 8a, 8b: LIQUID PREPARATIONS MADE FROM TRITURATIONS, AQUEOUS PREPARATIONS MADE FROM TRITURATIONS)
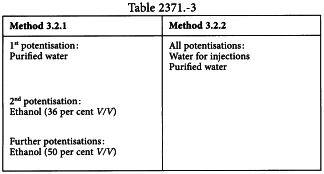
Preparations made according to Method 3.2.1 and Method 3.2.2 are produced from triturations D4, D5 and D6 or from triturations C4, C5 and C6, prepared according to method 4.1.1 by at least 2 potentisation steps.
Vehicles
The vehicles in Table 2371.-3 may be used.
Potentisation
For the first liquid potentisation, dissolve 1 part of the trituration in 9 parts (decimal dilutions) or 99 parts (centesimal dilutions) of the specified vehicle (see Table 2371.-3) and succuss. For further potentisations, proceed in the same manner with 1 part of the previous dilution.
The D6, D7, C6 and C7 dilutions produced by the above method are not to be used for the preparation of further dilutions. For the preparation of higher dilutions use the D8 or C8 dilution.
method 3.2.3
Preparations made according to Method 3.2.3 are produced from triturations D2 onwards and from triturations C1, C2, C3 and C4, prepared according to method 4.1.2.
Vehicles
Suitable vehicles such as ethanol of an appropriate concentration or purified water may be used.
Potentisation
Unless otherwise specified, the first liquid decimal dilution (Dn-1) is made from:
The following decimal dilution (Dn) is made from:
Subsequent decimal dilutions are produced as stated for Dn.
Unless otherwise specified, the first liquid centesimal dilution (Cn-1) is made from:
The following centesimal dilution (Cn) is made from:
Subsequent centesimal dilutions are produced as stated for Cn.
4. TRITURATIONS
METHOD 4.1
Method 4.1 is used for triturations, that is solid dilutions, of raw materials or of triturations prepared according to Methods 4.2.1 or 4.2.2. The duration and intensity of the trituration are such that homogeneity and potentisation are achieved.
Vehicle
Unless otherwise specified, lactose monohydrate is used.
METHOD 4.1.1 (HAB 6: TRITURATIONS)
Triturations are prepared manually or mechanically. Mechanical trituration must be used for quantities exceeding 1 kg. The resulting particle size of the raw material in the first decimal or centesimal dilution does not exceed 100 µm, unless otherwise prescribed in the individual monograph.
Ratios of raw material to vehicle
Where fresh plant material is used, the quantity of vehicle added is such so as to obtain 10 parts of the trituration (decimal trituration) or 100 parts of the trituration (centesimal trituration) from 1 part of the raw material (replace the mass of water lost from the fresh plant by an equivalent amount of the vehicle). A suitable gentle drying process may need to be applied to the solid dilution.
Where justified and authorised, it may be necessary to directly produce a C1 or ‘D2’ if to be used for further decimal triturations as the first solid trituration, made from 1 part of raw material and 99 parts of vehicle.
Trituration
Unless otherwise justified and authorised, the method consists of dividing the vehicle into 3 equal parts and adding the raw material to the first part, then adding the second and third part of the vehicle, thoroughly triturating after each addition.
For mechanical trituration, use a machine allowing the requirements for particle size of the first decimal or centesimal solid trituration to be met. A machine fitted with a scraping device may be used to ensure even trituration. The time required to prepare one trituration is at least 1 h, unless otherwise justified and authorised.
For manual trituration, divide the vehicle into 3 equal parts and briefly triturate the first part in a porcelain mortar. Add the raw material, triturate the mixture for 6 min, scrape down for 4 min with an appropriate non-metallic device (for example, a porcelain spatula). Triturate for a further 6 min, scrape down again for a further 4 min, then add the second part of the vehicle and continue as above. Proceed in the same manner with the rest of the vehicle. The minimum time required for the whole process is thus 1 h. Carry out the whole process again for each subsequent solid dilution.
Triturations from D5 or C5 onwards may also be prepared by intense mechanical treatment by a suitable mixing machine as follows: add the solid trituration to one third of the vehicle and mix. Add the second third of the vehicle, mix and proceed in the same manner with the last third of the vehicle. The whole process lasts minimum 1 hour, unless otherwise justified and authorised.
It is possible to change to a liquid medium from the 4th, 5th and 6th decimal or centesimal triturations, as described in Methods 3.2.1 and 3.2.2.
METHOD 4.1.2 (FRENCH PHARMACOPOEIA)
Trituration
Triturations are prepared as follows:
Decimal triturations
Reduce 1 part of the homoeopathic stock to a powder. Triturate carefully with a small quantity of the vehicle. Add the vehicle in small quantities until 9 parts of this vehicle have been used. The resulting trituration is the 1st decimal trituration (D1).
Triturate as described above 1 part of this trituration with 9 parts of the vehicle. The resulting trituration is the 2nd decimal trituration (D2).
In all cases, it is possible to change to a liquid medium after the 7th decimal trituration (D7) as described in Method 3.2.3.
Centesimal triturations
Proceed in the same manner but following a centesimal series.
In all cases, it is possible to change to a liquid medium after the 3rd centesimal trituration (C3) as described in Method 3.2.3.
METHOD 4.2
Method 4.2 is used for triturations, that is solid dilutions, of liquid preparations such as mother tinctures and solutions, their dilutions, mixtures and co-potentised mixtures.
Gradually impregnate the total amount of vehicle, gently dry the moist mixture, mill and sieve if necessary, then mix and triturate until homogeneity and potentisation are achieved. Trituration is further carried out as described for Method 4.1.1 or Method 4.1.2.
Vehicle
Unless otherwise specified, lactose monohydrate is used.
METHOD 4.2.1 (HAB 7: TRITURATIONS)
Ratios of starting material to vehicle
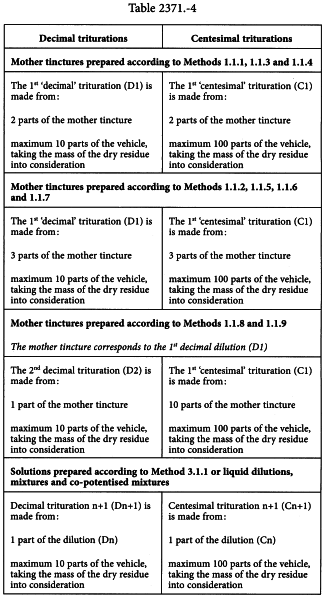
The quantity of vehicle added must always be such so as to obtain 10 parts of the trituration (decimal trituration) or 100 parts of the trituration (centesimal trituration) from the required number of parts of the liquid preparation (see Table 2371.-4), taking the mass of the dry residue into consideration. Where the dry residue is considered negligible, the quantity of vehicle added is 10 parts (decimal trituration) or 100 parts (centesimal trituration), for 1 part of the liquid preparation.
METHOD 4.2.2
Ratios of starting material to vehicle
5. other preparations
METHOD 5.1
Method 5.1 is used for preparing homoeopathic preparations by co-potentising 2 or more stocks and/or dilutions thereof, where co-potentisation consists of mixing several stocks or dilutions of stocks then potentising them together in one or more potentisation steps.
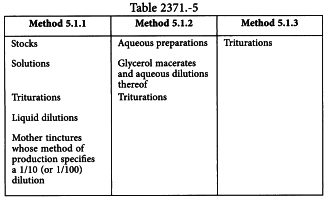
METHODS 5.1.1, 5.1.2, 5.1.3 (HAB 40a, 40b, 40c: CO-POTENTISED MIXTURES)
The stocks and/or dilutions in Table 2371.-5 may be used.
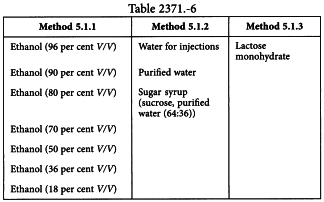
Vehicles
The choice of the vehicle is determined by and must comply with any special requirement for the particular stock as well as the dosage form (see table Table 2371.-6).
For Method 5.1.1, when starting from a trituration and where justified, purified water is used for the 1st potentisation step.
For Method 5.1.2, when starting from a glycerol macerate containing sodium chloride, unless otherwise justified and authorised, the following vehicle is used: 0.2 parts of sodium hydrogen carbonate and 8.8 parts of sodium chloride in 991 parts of water for injections.
Potentisation
For each potentisation step, combine and succuss or triturate 1 part of the given mixture with 9 parts (decimal dilutions) or 99 parts (centesimal dilutions) of the appropriate vehicle.
METHOD 5.1.4
Vehicles
Ethanol of an appropriate concentration, purified water or lactose monohydrate may, for example, be used.
Potentisation
Potentisation may be performed as prescribed for Methods 5.1.1, 5.1.2 and 5.1.3, either on the last step or on several successive steps.
METHOD 5.1.5
Vehicle
Ethanol of an appropriate concentration, purified water or lactose monohydrate may, for example, be used.
Potentisation
For a co-potentisation of centesimal dilutions, each dilution (Cn-1) represents 1 per cent of the final product and the proportion of vehicle to be added is reduced by the proportion of the active substances [i.e. 100 per cent -(1 per cent × the number of active substances)]. The same procedure applies, in the appropriate proportions, when co-potentising decimal dilutions.
METHOD 5.2
Method 5.2 is used to prepare potencies with a 50 000 dilution factor (LM) by alternate steps of liquid dilution and impregnation of sucrose pillules (category 1, unless otherwise authorised).
Solid and liquid potencies
Use a dropper (2.1.1).
To prepare pillules of 1st LM potency, a C3 trituration of the substance to be potentised is processed as follows: dissolve 60 mg of the C3 trituration in a volume of ethanol (18 per cent V/V) or another authorised concentration, corresponding to 500 drops. To 1 drop of this solution add a volume of ethanol (90 per cent V/V) or another authorised concentration, corresponding to 100 drops and succuss at least 100 times. Use all of this solution to impregnate a mass of pillules corresponding to 50 000 pillules, then allow to dry in air.
To prepare pillules of 2nd LM potency, process pillules of 1st LM potency as follows: dissolve 1 pillule of 1st LM potency in 1 drop of purified water, add a volume of ethanol (90 per cent V/V) or another authorised concentration, corresponding to 100 drops and succuss at least 100 times. Use all of this solution to impregnate a mass of pillules corresponding to 50 000 pillules, then allow to dry in air.
Subsequent solid potencies are prepared as for the 2nd LM potency.
Liquid potencies (HAB 17)
To prepare a liquid LM potency, dissolve 1 pillule of the required potency in 10.0 mL of ethanol (18 per cent V/V) or another authorised concentration. The potency of the solution corresponds to the potency of the pillule dissolved therein.
Ph Eur