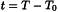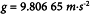Part III
GENERAL NOTICES OF THE EUROPEAN PHARMACOPOEIA
1.1. General statements
The General Notices apply to all monographs and other texts of the European Pharmacopoeia.
The official texts of the European Pharmacopoeia are published in English and French. Translations in other languages may be prepared by the signatory States of the European Pharmacopoeia Convention. In case of doubt or dispute, the English and French versions are alone authoritative.
In the texts of the European Pharmacopoeia, the word ‘Pharmacopoeia’ without qualification means the European Pharmacopoeia. The official abbreviation Ph. Eur. may be used to indicate the European Pharmacopoeia.
The use of the title or the subtitle of a monograph implies that the article complies with the requirements of the relevant monograph. Such references to monographs in the texts of the Pharmacopoeia are shown using the monograph title and reference number in italics.
A preparation must comply throughout its period of validity; a distinct period of validity and/or specifications for opened or broached containers may be decided by the competent authority. The subject of any other monograph must comply throughout its period of use. The period of validity that is assigned to any given article and the time from which that period is to be calculated are decided by the competent authority in light of experimental results of stability studies.
Unless otherwise indicated in the General Notices or in the monographs, statements in monographs constitute mandatory requirements. General chapters become mandatory when referred to in a monograph, unless such reference is made in a way that indicates that it is not the intention to make the text referred to mandatory but rather to cite it for information.
The active substances, excipients, pharmaceutical preparations and other articles described in the monographs are intended for human and veterinary use (unless explicitly restricted to one of these uses).
Quality systems
The quality standards represented by monographs are valid only where the articles in question are produced within the framework of a suitable quality system. The quality system must assure that the articles consistently meet the requirements of the Pharmacopoeia.
Alternative methods
The tests and assays described are the official methods upon which the standards of the Pharmacopoeia are based. With the agreement of the competent authority, alternative methods of analysis may be used for control purposes, provided that the methods used enable an unequivocal decision to be made as to whether compliance with the standards of the monographs would be achieved if the official methods were used. In the event of doubt or dispute, the methods of analysis of the Pharmacopoeia are alone authoritative.
Demonstration of compliance with the Pharmacopoeia
(1) An article is not of Pharmacopoeia quality unless it complies with all the requirements stated in the monograph. This does not imply that performance of all the tests in a monograph is necessarily a prerequisite for a manufacturer in assessing compliance with the Pharmacopoeia before release of a product. The manufacturer may obtain assurance that a product is of Pharmacopoeia quality on the basis of its design, together with its control strategy and data derived, for example, from validation studies of the manufacturing process.
(2) An enhanced approach to quality control could utilise process analytical technology (PAT) and/or real-time release testing (including parametric release) strategies as alternatives to end-product testing alone. Real-time release testing in circumstances deemed appropriate by the competent authority is thus not precluded by the need to comply with the Pharmacopoeia.
(3) Reduction of animal testing: the European Pharmacopoeia is dedicated to phasing out the use of animals for test purposes, in accordance with the 3Rs (Replacement, Reduction, Refinement) set out in the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes. In demonstrating compliance with the Pharmacopoeia as indicated above (1), manufacturers may consider establishing additional systems to monitor consistency of production. With the agreement of the competent authority, the choice of tests performed to assess compliance with the Pharmacopoeia when animal tests are prescribed is established in such a way that animal usage is minimised as much as possible.
Grade of materials
Certain materials that are the subject of a pharmacopoeial monograph may exist in different grades suitable for different purposes. Unless otherwise indicated in the monograph, the requirements apply to all grades of the material. In some monographs, particularly those on excipients, a list of functionality-related characteristics that are relevant to the use of the substance may be appended to the monograph for information. Test methods for determination of one or more of these characteristics may be given, also for information.
General monographs
Substances and preparations that are the subject of an individual monograph are also required to comply with relevant, applicable general monographs. Cross-references to applicable general monographs are not normally given in individual monographs.
General monographs apply to all substances and preparations within the scope of the Definition section of the general monograph, except where a preamble limits the application, for example to substances and preparations that are the subject of a monograph of the Pharmacopoeia.
General monographs on dosage forms apply to all preparations of the type defined. The requirements are not necessarily comprehensive for a given specific preparation and requirements additional to those prescribed in the general monograph may be imposed by the competent authority.
General monographs and individual monographs are complementary. If the provisions of a general monograph do not apply to a particular product, this is expressly stated in the individual monograph.
Validation of pharmacopoeial methods
The test methods given in monographs and general chapters have been validated in accordance with accepted scientific practice and current recommendations on analytical validation. Unless otherwise stated in the monograph or general chapter, validation of the test methods by the analyst is not required.
Implementation of pharmacopoeial methods
When implementing a pharmacopoeial method, the user must assess whether and to what extent the suitability of the method under the actual conditions of use needs to be demonstrated according to relevant monographs, general chapters and quality systems.
Conventional terms
The term ‘competent authority’ means the national, supranational or international body or organisation vested with the authority for making decisions concerning the issue in question. It may, for example, be a national pharmacopoeia authority, a licensing authority or an official control laboratory.
The expression ‘unless otherwise justified and authorised’ means that the requirements have to be met, unless the competent authority authorises a modification or an exemption where justified in a particular case.
Statements containing the word ‘should’ are informative or advisory.
In certain monographs or other texts, the terms ‘suitable’ and ‘appropriate’ are used to describe a reagent, micro-organism, test method etc.; if criteria for suitability are not described in the monograph, suitability is demonstrated to the satisfaction of the competent authority.
Medicinal product (a) Any substance or combination of substances presented as having properties for treating or preventing disease in human beings and/or animals; or (b) any substance or combination of substances that may be used in or administered to human beings and/or animals with a view either to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or to making a medical diagnosis.
Herbal medicinal product Any medicinal product, exclusively containing as active ingredients one or more herbal drugs or one or more herbal drug preparations, or one or more such herbal drugs in combination with one or more such herbal drug preparations.
Active substance Any substance intended to be used in the manufacture of a medicinal product and that, when so used, becomes an active ingredient of the medicinal product. Such substances are intended to furnish a pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment or prevention of disease, or to affect the structure and function of the body.
Excipient (auxiliary substance). Any constituent of a medicinal product that is not an active substance. Adjuvants, stabilisers, antimicrobial preservatives, diluents, antioxidants, for example, are excipients.
Interchangeable methods
Certain general chapters contain a statement that the text in question is harmonised with the corresponding text of the Japanese Pharmacopoeia and/or the United States Pharmacopeia and that these texts are interchangeable. This implies that if a substance or preparation is found to comply with a requirement using an interchangeable method from one of these pharmacopoeias it complies with the requirements of the European Pharmacopoeia. In the event of doubt or dispute, the text of the European Pharmacopoeia is alone authoritative.
References to regulatory documents
Monographs and general chapters may contain references to documents issued by regulatory authorities for medicines, for example directives and notes for guidance of the European Union. These references are provided for information for users for the Pharmacopoeia. Inclusion of such a reference does not modify the status of the documents referred to, which may be mandatory or for guidance.
1.2. Other provisions applying to general chapters and monographs
Quantities
In tests with numerical limits and assays, the quantity stated to be taken for examination is approximate. The amount actually used, which may deviate by not more than 10 per cent from that stated, is accurately weighed or measured and the result is calculated from this exact quantity. In tests where the limit is not numerical, but usually depends upon comparison with the behaviour of a reference substance in the same conditions, the stated quantity is taken for examination. Reagents are used in the prescribed amounts.
Quantities are weighed or measured with an accuracy commensurate with the indicated degree of precision. For weighings, the precision corresponds to plus or minus 5 units after the last figure stated (for example, 0.25 g is to be interpreted as 0.245 g to 0.255 g). For the measurement of volumes, if the figure after the decimal point is a zero or ends in a zero (for example, 10.0 mL or 0.50 mL), the volume is measured using a pipette, a volumetric flask or a burette, as appropriate; otherwise, a graduated measuring cylinder or a graduated pipette may be used. Volumes stated in microlitres are measured using a micropipette or microsyringe.
It is recognised, however, that in certain cases the precision with which quantities are stated does not correspond to the number of significant figures stated in a specified numerical limit. The weighings and measurements are then carried out with a sufficiently improved accuracy.
Apparatus and procedures
Volumetric glassware complies with Class A requirements of the appropriate International Standard issued by the International Organisation for Standardisation.
Unless otherwise prescribed, analytical procedures are carried out at a temperature between 15 °C and 25 °C.
Unless otherwise prescribed, comparative tests are carried out using identical tubes of colourless, transparent, neutral glass with a flat base; the volumes of liquid prescribed are for use with tubes having an internal diameter of 16 mm, but tubes with a larger internal diameter may be used provided the volume of liquid used is adjusted (2.1.5). Equal volumes of the liquids to be compared are examined down the vertical axis of the tubes against a white background, or if necessary against a black background. The examination is carried out in diffuse light.
Any solvent required in a test or assay in which an indicator is to be used is previously neutralised to the indicator, unless a blank test is prescribed.
Water-bath
The term ‘water-bath’ means a bath of boiling water unless water at another temperature is indicated. Other methods of heating may be substituted provided the temperature is near to but not higher than 100 °C or the indicated temperature.
Drying and ignition to constant mass
The terms ‘dried to constant mass’ and ‘ignited to constant mass’ mean that 2 consecutive weighings do not differ by more than 0.5 mg, the 2nd weighing following an additional period of drying or of ignition respectively appropriate to the nature and quantity of the residue.
Where drying is prescribed using one of the expressions ‘in a desiccator’ or ‘in vacuo’, it is carried out using the conditions described in chapter 2.2.32. Loss on drying.
Reagents
The proper conduct of the analytical procedures described in the Pharmacopoeia and the reliability of the results depend, in part, upon the quality of the reagents used. The reagents are described in general chapter 4. It is assumed that reagents of analytical grade are used; for some reagents, tests to determine suitability are included in the specifications.
Solvents
Where the name of the solvent is not stated, the term ‘solution’ implies a solution in water.
Where the use of water is specified or implied in the analytical procedures described in the Pharmacopoeia or for the preparation of reagents, water complying with the requirements of the monograph Purified water (0008) is used, except that for many purposes the requirements for bacterial endotoxins (Purified water in bulk) and microbial contamination (Purified water in containers) are not relevant. The term ‘distilled water’ indicates purified water prepared by distillation.
The term ‘ethanol’ without qualification means anhydrous ethanol. The term ‘alcohol’ without qualification means ethanol (96 per cent). Other dilutions of ethanol are indicated by the term ‘ethanol’ or ‘alcohol’ followed by a statement of the percentage by volume of ethanol (C2H6O) required.
Expression of content
In defining content, the expression ‘per cent’ is used according to circumstances with one of 2 meanings:
The expression ‘parts per million’ (or ppm) refers to mass in mass, unless otherwise specified.
Temperature
Where an analytical procedure describes temperature without a figure, the general terms used have the following meaning:
1.3. General chapters
Containers
Materials used for containers are described in general chapter 3.1. General names used for materials, particularly plastic materials, each cover a range of products varying not only in the properties of the principal constituent but also in the additives used. The test methods and limits for materials depend on the formulation and are therefore applicable only for materials whose formulation is covered by the preamble to the specification. The use of materials with different formulations, and the test methods and limits applied to them, are subject to agreement by the competent authority.
The specifications for containers in general chapter 3.2 have been developed for general application to containers of the stated category, but in view of the wide variety of containers available and possible new developments, the publication of a specification does not exclude the use, in justified circumstances, of containers that comply with other specifications, subject to agreement by the competent authority.
Reference may be made within the monographs of the Pharmacopoeia to the definitions and specifications for containers provided in chapter 3.2. Containers. The general monographs for pharmaceutical dosage forms may, under the heading Definition/Production, require the use of certain types of container; certain other monographs may, under the heading Storage, indicate the type of container that is recommended for use.
1.4. Monographs
Titles
Monograph titles are in English and French in the respective versions and there is a Latin subtitle.
Relative Atomic and Molecular Masses
The relative atomic mass (Ar) or the relative molecular mass (Mr) is shown, as and where appropriate, at the beginning of each monograph. The relative atomic and molecular masses and the molecular and graphic formulae do not constitute analytical standards for the substances described.
Chemical Abstracts Service (CAS) Registry Number
CAS registry numbers are included for information in monographs, where applicable, to provide convenient access to useful information for users. CAS Registry Number® is a registered trademark of the American Chemical Society.
Definition
Statements under the heading Definition constitute an official definition of the substance, preparation or other article that is the subject of the monograph.
Limits of content Where limits of content are prescribed, they are those determined by the method described under Assay.
Herbal drugs In monographs on herbal drugs, the definition indicates whether the subject of the monograph is, for example, the whole drug or the drug in powdered form. Where a monograph applies to the drug in several states, for example both to the whole drug and the drug in powdered form, the definition states this.
Production
Statements under the heading Production draw attention to particular aspects of the manufacturing process but are not necessarily comprehensive. They constitute mandatory requirements for manufacturers, unless otherwise stated. They may relate, for example, to source materials; to the manufacturing process itself and its validation and control; to in-process testing; or to testing that is to be carried out by the manufacturer on the final article, either on selected batches or on each batch prior to release. These statements cannot necessarily be verified on a sample of the final article by an independent analyst. The competent authority may establish that the instructions have been followed, for example, by examination of data received from the manufacturer, by inspection of manufacture or by testing appropriate samples.
The absence of a Production section does not imply that attention to features such as those referred to above is not required.
Choice of vaccine strain, Choice of vaccine composition The Production section of a monograph may define the characteristics of a vaccine strain or vaccine composition. Unless otherwise stated, test methods given for verification of these characteristics are provided for information as examples of suitable methods. Subject to approval by the competent authority, other test methods may be used without validation against the method shown in the monograph.
Potential Adulteration
Due to the increasing number of fraudulent activities and cases of adulteration, information may be made available to Ph. Eur. users to help detect adulterated materials (i.e. active substances, excipients, intermediate products, bulk products and finished products).
To this purpose, a method for the detection of potential adulterants and relevant limits, together with a reminder that all stages of production and sourcing are subjected to a suitable quality system, may be included in this section of monographs on substances for which an incident has occurred or that present a risk of deliberate contamination. The frequency of testing by manufacturers or by users (e.g. manufacturers of intermediate products, bulk products and finished products, where relevant) depends on a risk assessment, taking into account the level of knowledge of the whole supply chain and national requirements.
This section constitutes requirements for the whole supply chain, from manufacturers to users (e.g. manufacturers of intermediate products, bulk products and finished products, where relevant). The absence of this section does not imply that attention to features such as those referred to above is not required.
Characters
The statements under the heading Characters are not to be interpreted in a strict sense and are not requirements.
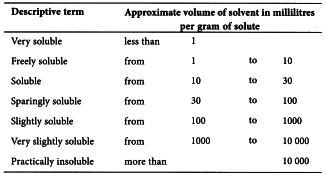
Solubility In statements of solubility in the Characters section, the terms used have the following significance, referred to a temperature between 15 °C and 25 °C.
The term ‘partly soluble’ is used to describe a mixture where only some of the components dissolve. The term ‘miscible’ is used to describe a liquid that is miscible in all proportions with the stated solvent.
Identification
Scope The tests given in the Identification section are not designed to give a full confirmation of the chemical structure or composition of the product; they are intended to give confirmation, with an acceptable degree of assurance, that the article conforms to the description on the label.
First and second identifications Certain monographs have subdivisions entitled ‘First identification’ and ‘Second identification’. The test or tests that constitute the ‘First identification’ may be used in all circumstances. The test or tests that constitute the ‘Second identification’ may be used in pharmacies provided it can be demonstrated that the substance or preparation is fully traceable to a batch certified to comply with all the other requirements of the monograph.
Certain monographs give two or more sets of tests for the purpose of the first identification, which are equivalent and may be used independently. One or more of these sets usually contain a cross-reference to a test prescribed in the Tests section of the monograph. It may be used to simplify the work of the analyst carrying out the identification and the prescribed tests. For example, one identification set cross-refers to a test for enantiomeric purity while the other set gives a test for specific optical rotation: the intended purpose of the two is the same, that is, verification that the correct enantiomer is present.
Powdered herbal drugs Monographs on herbal drugs may contain schematic drawings of the powdered drug. These drawings complement the description given in the relevant identification test.
Tests and Assays
Scope The requirements are not framed to take account of all possible impurities. It is not to be presumed, for example, that an impurity that is not detectable by means of the prescribed tests is tolerated if common sense and good pharmaceutical practice require that it be absent. See also below under Impurities.
Calculation Where the result of a test or assay is required to be calculated with reference to the dried or anhydrous substance or on some other specified basis, the determination of loss on drying, water content or other property is carried out by the method prescribed in the relevant test in the monograph. The words ‘dried substance’ or ‘anhydrous substance’ etc. appear in parentheses after the result.Where a quantitative determination of a residual solvent is carried out and a test for loss on drying is not carried out, the content of residual solvent is taken into account for the calculation of the assay content of the substance, the specific optical rotation and the specific absorbance. No further indication is given in the specific monograph.
Limits The limits prescribed are based on data obtained in normal analytical practice; they take account of normal analytical errors, of acceptable variations in manufacture and compounding and of deterioration to an extent considered acceptable. No further tolerances are to be applied to the limits prescribed to determine whether the article being examined complies with the requirements of the monograph.
In determining compliance with a numerical limit, the calculated result of a test or assay is first rounded to the number of significant figures stated, unless otherwise prescribed. The limits, regardless of whether the values are expressed as percentages or as absolute values, are considered significant to the last digit shown (for example 140 indicates 3 significant figures). The last figure of the result is increased by one when the part rejected is equal to or exceeds one half-unit, whereas it is not modified when the part rejected is less than a half-unit.
Indication of permitted limit of impurities The acceptance criteria for related substances are expressed in monographs either in terms of comparison of peak areas (comparative tests) or as numerical values. For comparative tests, the approximate content of impurity tolerated, or the sum of impurities, may be indicated in brackets for information only. Acceptance or rejection is determined on the basis of compliance or non-compliance with the stated test. If the use of a reference substance for the named impurity is not prescribed, this content may be expressed as a nominal concentration of the substance used to prepare the reference solution specified in the monograph, unless otherwise described.
Herbal drugs For herbal drugs, the sulfated ash, total ash, water-soluble matter, alcohol-soluble matter, water content, content of essential oil and content of active principle are calculated with reference to the drug that has not been specially dried, unless otherwise prescribed in the monograph.
Equivalents Where an equivalent is given, for the purposes of the Pharmacopoeia only the figures shown are to be used in applying the requirements of the monograph.
Culture media The culture media described in monographs and general chapters have been found to be satisfactory for the intended purpose. However, the components of media, particularly those of biological origin, are of variable quality, and it may be necessary for optimal performance to modulate the concentration of some ingredients, notably:
Storage
The information and recommendations given under the heading Storage do not constitute a pharmacopoeial requirement but the competent authority may specify particular storage conditions that must be met.
The articles described in the Pharmacopoeia are stored in such a way as to prevent contamination and, as far as possible, deterioration. Where special conditions of storage are recommended, including the type of container (see section 1.3. General chapters) and limits of temperature, they are stated in the monograph.
The following expressions are used in monographs under Storage with the meaning shown.
In an airtight container Means that the product is stored in an airtight container (3.2). Care is to be taken when the container is opened in a damp atmosphere. A low moisture content may be maintained, if necessary, by the use of a desiccant in the container provided that direct contact with the product is avoided.
Protected from light Means that the product is stored either in a container made of a material that absorbs actinic light sufficiently to protect the contents from change induced by such light, or in a container enclosed in an outer cover that provides such protection, or is stored in a place from which all such light is excluded.
Labelling
In general, labelling of medicines is subject to supranational and national regulation and to international agreements. The statements under the heading Labelling are not therefore comprehensive and, moreover, for the purposes of the Pharmacopoeia only those statements that are necessary to demonstrate compliance or non-compliance with the monograph are mandatory. Any other labelling statements are included as recommendations. When the term ‘label’ is used in the Pharmacopoeia, the labelling statements may appear on the container, the package, a leaflet accompanying the package, or a certificate of analysis accompanying the article, as decided by the competent authority.
Warnings
Materials described in monographs and reagents specified for use in the Pharmacopoeia may be injurious to health unless adequate precautions are taken. The principles of good quality control laboratory practice and the provisions of any appropriate regulations are to be observed at all times. Attention is drawn to particular hazards in certain monographs by means of a warning statement; absence of such a statement is not to be taken to mean that no hazard exists.
Impurities
A list of all known and potential impurities that have been shown to be detected by the tests in a monograph may be given. See also chapter 5.10. Control of impurities in substances for pharmaceutical use. The impurities are designated by a letter or letters of the alphabet. Where a letter appears to be missing, the impurity designated by this letter has been deleted from the list during monograph development prior to publication or during monograph revision.
Functionality-Related Characteristics of Excipients
Monographs on excipients may have a section on functionality-related characteristics. The characteristics, any test methods for determination and any tolerances are not mandatory requirements; they may nevertheless be relevant for use of the excipient and are given for information (see also section 1.1. General statements).
Reference Standards
Certain monographs require the use of reference standards (chemical reference substances, herbal reference standards, biological reference preparations, reference spectra). See also chapter 5.12. Reference standards. The European Pharmacopoeia Commission establishes the official reference standards, which are alone authoritative in case of arbitration. These reference standards are available from the European Directorate for the Quality of Medicines & HealthCare (EDQM). Information on the available reference standards and a batch validity statement can be obtained via the EDQM website.
1.5. Abbreviations and symbols
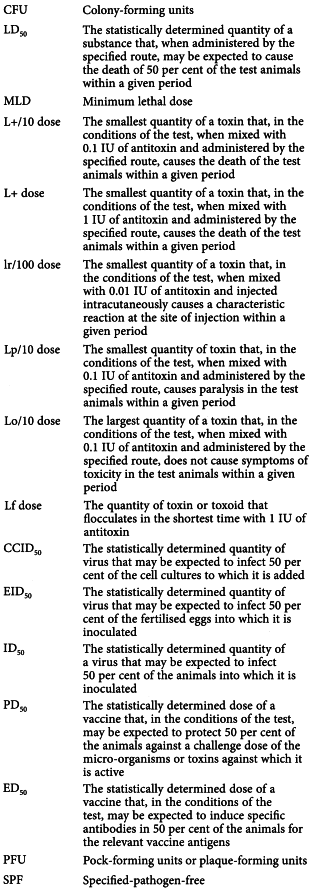
Abbreviations used in the monographs on immunoglobulins, immunosera and vaccines
Collections of micro-organisms
1.6. Units of the international system (si) used in the pharmacopoeia and equivalence with other units
INTERNATIONAL SYSTEM OF UNITS (SI)
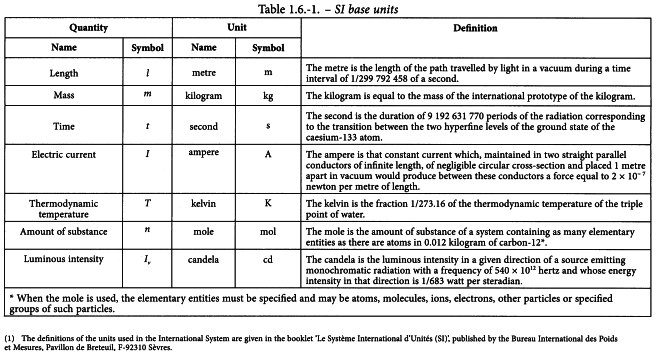
The International System of Units comprises 3 classes of units, namely base units, derived units and supplementary units1. The base units and their definitions are set out in Table 1.6-1.
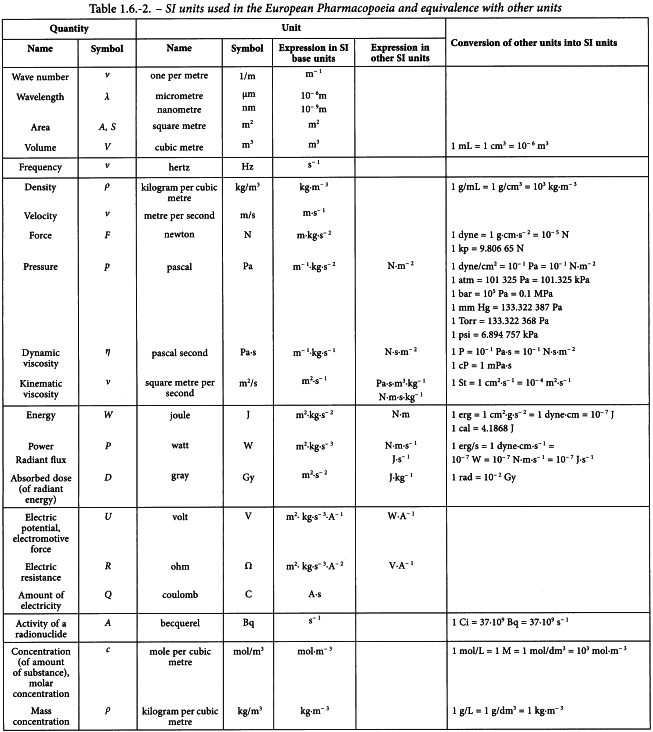
The derived units may be formed by combining the base units according to the algebraic relationships linking the corresponding quantities. Some of these derived units have special names and symbols. The SI units used in the Pharmacopoeia are shown in Table 1.6-2.
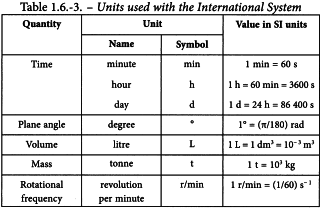
Some important and widely used units outside the International System are shown in Table 1.6-3.
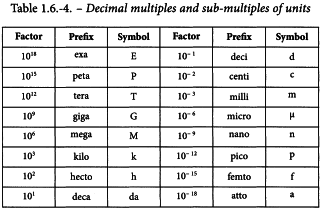
The prefixes shown in Table 1.6-4 are used to form the names and symbols of the decimal multiples and submultiples of SI units.
NOTES
In the Pharmacopoeia, the Celsius temperature is used (symbol t). This is defined by the following equation:
where T0 = 273.15 K by definition. The Celsius or centigrade temperature is expressed in degrees Celsius (symbol °C). The unit ‘degree Celsius’ is equal to the unit ‘kelvin’.
2. The practical expressions of concentrations used in the Pharmacopoeia are defined in the General Notices.
3. The radian is the plane angle between two radii of a circle that cut off on the circumference an arc equal in length to the radius.
4. In the Pharmacopoeia, conditions of centrifugation are defined by reference to the acceleration due to gravity (g):
5. Certain quantities without dimensions are used in the Pharmacopoeia: relative density (2.2.5), absorbance (2.2.25), specific absorbance (2.2.25) and refractive index (2.2.6).
6. The microkatal is defined as the enzymic activity that, under defined conditions, produces the transformation (e.g. hydrolysis) of 1 micromole of the substrate per second.