SC I C. Bacterial Endotoxin Testing
This section provides an exposition of the Commission’s policy and information on its implementation. The guidelines of the European Pharmacopoeia are included as an Annex.
The test for bacterial endotoxins of the European Pharmacopoeia (Ph Eur) is included as Appendix XIV C of the British Pharmacopoeia. This text has been prepared in collaboration with the Japanese Pharmacopoeia and the United States Pharmacopeia.
1. This in vitro test is being progressively applied in appropriate monographs of both the British and European Pharmacopoeia in place of the in vivo test for pyrogens.
2. Methods for the detection of Gram-negative bacterial endotoxins are based on the use of a lysate of amoebocytes from the horseshoe crab (Limulus polyphemus or Tachypleus tridentatus). Addition of endotoxin to this lysate may result in gelation, precipitation or turbidity. The method used in the monographs of the European and British Pharmacopoeias is, unless otherwise stated, that using a gelation end-point.
3. A European Pharmacopoeia Biological Reference Preparation (BRP) of Endotoxin calibrated in International Units (IU) has been established for use in this test. The current BRP (batch 5) was established following an international collaborative study. It consists of endotoxin from the same bulk as the Third International Standard established by the World Health Organization and the current standard established by the Food and Drug Administration and the United States Pharmacopeia for use in the United States of America (EC-7). Following adoption of the recommendations in the report of the collaborative study1 global harmonisation of endotoxin unitage has been maintained2, that is, the FDA/USP Endotoxin Unit (EU) is equivalent to the International Unit (IU).
General policy
4. In any individual monograph only one test is required, either that for pyrogens or that for bacterial endotoxins.
5. In the absence of evidence to the contrary, the test for bacterial endotoxins is preferred, since it is considered usually to provide equal or better protection to the patient.
6. Before including a test for bacterial endotoxins in a monograph, evidence is required that a test, as described in Appendix XIV C, can be applied satisfactorily to the item in question.
7. The necessary information is sought from manufacturers. Companies are invited to provide any validation data that they have concerning the applicability of the test for bacterial endotoxins to the substances and formulations of interest.
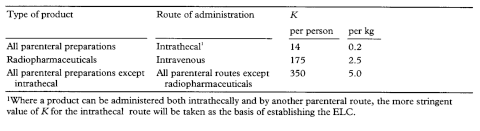
8. In order to set an appropriate limit for bacterial endotoxins it is necessary to know the intended route of parenteral administration (in particular, if the substance may be administered intrathecally) together with the maximum dose as recommended in relevant product data sheets. The limit for a given material or preparation is expressed as the endotoxin limit (EL) or endotoxin limit concentration (EL). The EL may be expressed in IU of endotoxin per millilitre for a defined solution of the material or preparation, or, for some medicinal substances, in IU of endotoxin in relation to a defined quantity of the material (that is, per milligram or, for biologically assayed materials, per IU) of material. The limit, which is stated in the monograph, is usually established on the following basis:
EL = K/M
Implementation
9. The British Pharmacopoeia Commission is seeking to replace the test for pyrogens by that for bacterial endotoxins wherever possible in the Pharmacopoeia. The European Pharmacopoeia Commission has a similar policy and has indicated that, for monographs already published, the change will be carried out, where appropriate, whenever the monograph in question is revised.
Annex
Guidelines concerning the test for Bacterial endotoxins have been published as an Annex to the method text 2.6.14 in the European Pharmacopoeia and are reproduced here.
The following section is published for information.