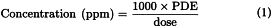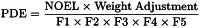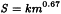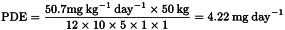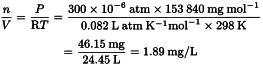SC IV D. Residual Solvents
LIMITING RESIDUAL SOLVENT LEVELS IN ACTIVE SUBSTANCES, EXCIPIENTS AND MEDICINAL PRODUCTS
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has adopted Impurities Guidelines for Residual Solvents which prescribes limits for the content of solvents which may remain in active substances, excipients and medicinal products after processing. This guideline, the text of which is reproduced below, excludes existing marketed products. The European Pharmacopoeia is, however, applying the same principles enshrined in the guideline to existing active substances, excipients and medicinal products whether or not they are the subject of a monograph of the Pharmacopoeia. All substances and products are to be tested for the content of solvents likely to be present in a substance or product.
Where the limits to be applied comply with those given below, tests for residual solvents are not generally mentioned in specific monographs since the solvents employed may vary from one manufacturer to another and the requirements of this general chapter are applied via the general monograph on Substances for Pharmaceutical Use (2034). The competent authority is to be informed of the solvents employed during the production process. This information is also given in the dossier submitted for a certificate of suitability of the monographs of the European Pharmacopoeia and is mentioned on the certificate.
Where only Class 3 solvents are used, a test for loss on drying may be applied or a specific determination of the solvent may be made. If for a Class 3 solvent a justified and authorised limit higher than 0.5 per cent is applied, a specific determination of the solvent is required.
When Class 1 residual solvents or Class 2 residual solvents (or Class 3 residual solvents which exceed the 0.5 per cent) are used, the methodology described in the general method (2.4.24) is to be applied wherever possible. Otherwise an appropriate validated method is to be employed.
When a quantitative determination of a residual solvent is carried out, the result is taken into account for the calculation of the content of the substance except where a test for drying is carried out.
IMPURITIES: GUIDELINES FOR RESIDUAL SOLVENTS (CPMP/ICH/82 260/06)
1. INTRODUCTION
2. SCOPE OF THE GUIDELINE
3. GENERAL PRINCIPLES
4. LIMITS OF RESIDUAL SOLVENTS
GLOSSARY
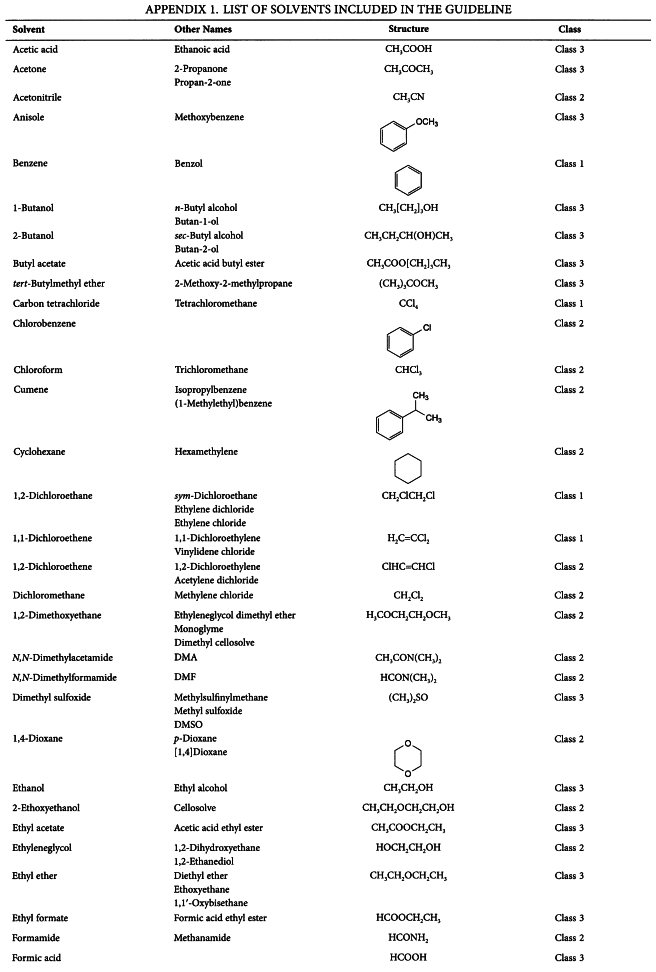
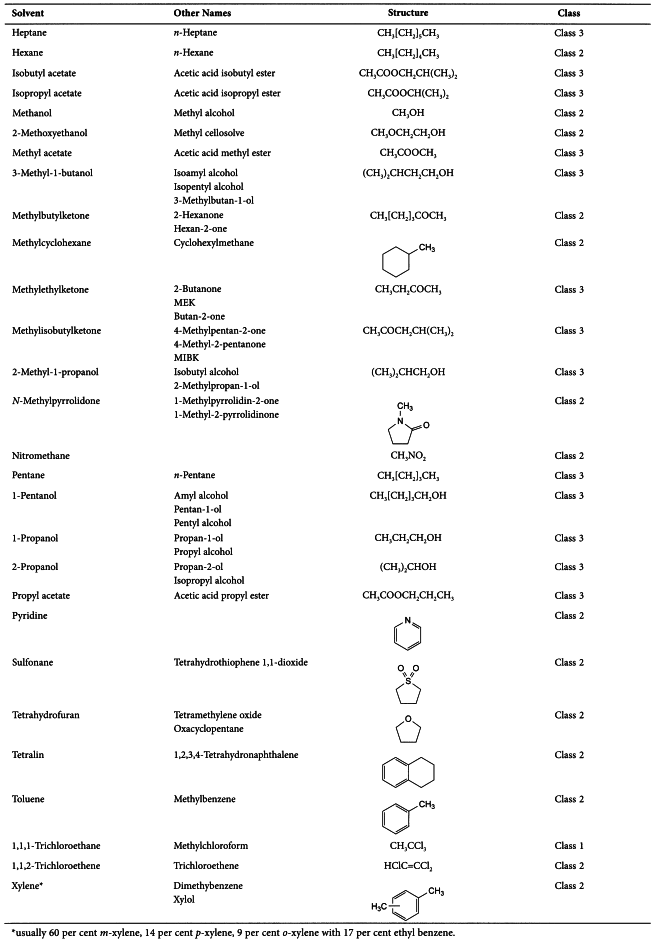
APPENDIX 1. LIST OF SOLVENTS INCLUDED IN THE GUIDELINE
APPENDIX 2. ADDITIONAL BACKGROUND
APPENDIX 3. METHODS FOR ESTABLISHING EXPOSURE LIMITS
1. INTRODUCTION
The objective of this guideline is to recommend acceptable amounts of residual solvents in pharmaceuticals for the safety of the patient. The guideline recommends the use of less toxic solvents and describes levels considered to be toxicologically acceptable for some residual solvents.
Residual solvents in pharmaceuticals are defined here as organic volatile chemicals that are used or produced in the manufacture of active substances or excipients, or in the preparation of medicinal products. The solvents are not completely removed by practical manufacturing techniques. Appropriate selection of the solvent for the synthesis of active substance may enhance the yield, or determine characteristics such as crystal form, purity, and solubility. Therefore, the solvent may sometimes be a critical parameter in the synthetic process. This guideline does not address solvents deliberately used as excipients nor does it address solvates. However, the content of solvents in such products should be evaluated and justified.
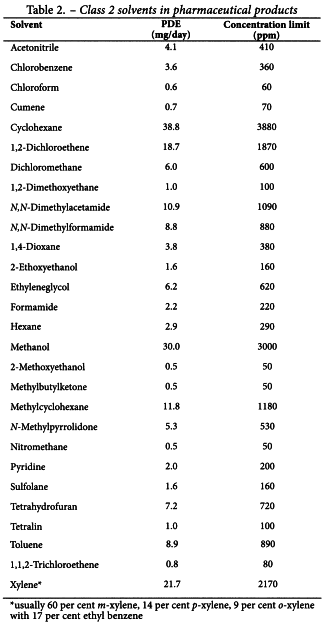
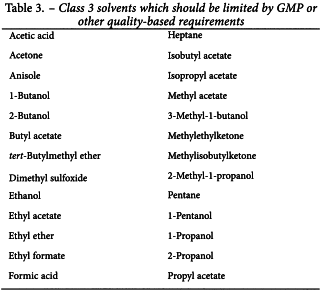
Since there is no therapeutic benefit from residual solvents, all residual solvents should be removed to the extent possible to meet product specifications, good manufacturing practices, or other quality-based requirements. Medicinal products should contain no higher levels of residual solvents than can be supported by safety data. Some solvents that are known to cause unacceptable toxicities (Class 1, Table 1) should be avoided in the production of active substances, excipients, or medicinal products unless their use can be strongly justified in a risk-benefit assessment. Some solvents associated with less severe toxicity (Class 2, Table 2) should be limited in order to protect patients from potential adverse effects. Ideally, less toxic solvents (Class 3, Table 3) should be used where practical. The complete list of solvents included in this guideline is given in Appendix 1.
The lists are not exhaustive and other solvents can be used and later added to the lists. Recommended limits of Class 1 and 2 solvents or classification of solvents may change as new safety data becomes available. Supporting safety data in a marketing application for a new medicinal product containing a new solvent may be based on concepts in this guideline or the concept of qualification of impurities as expressed in the guideline for active substances (Q3A, Impurities in New Active Substances) or medicinal products (Q3B, Impurities in New Medicinal Products), or all three guidelines.
2. SCOPE OF THE GUIDELINE
Residual solvents in active substances, excipients, and in medicinal products are within the scope of this guideline. Therefore, testing should be performed for residual solvents when production or purification processes are known to result in the presence of such solvents. It is only necessary to test for solvents that are used or produced in the manufacture or purification of active substances, excipients, or medicinal product. Although manufacturers may choose to test the medicinal product, a cumulative method may be used to calculate the residual solvent levels in the medicinal product from the levels in the ingredients used to produce the medicinal product. If the calculation results in a level equal to or below that recommended in this guideline, no testing of the medicinal product for residual solvents need be considered. If however, the calculated level is above the recommended level, the medicinal product should be tested to ascertain whether the formulation process has reduced the relevant solvent level to within the acceptable amount. Medicinal product should also be tested if a solvent is used during its manufacture.
This guideline does not apply to potential new active substances, excipients, or medicinal products used during the clinical research stages of development, nor does it apply to existing marketed medicinal products.
The guideline applies to all dosage forms and routes of administration. Higher levels of residual solvents may be acceptable in certain cases such as short term (30 days or less) or topical application. Justification for these levels should be made on a case by case basis.
See Appendix 2 for additional background information related to residual solvents.
3. GENERAL PRINCIPLES
3.1. CLASSIFICATION OF RESIDUAL SOLVENTS BY RISK ASSESSMENT
The term “tolerable daily intake” (TDI) is used by the International Program on Chemical Safety (IPCS) to describe exposure limits of toxic chemicals and “acceptable daily intake” (ADI) is used by the World Health Organization (WHO) and other national and international health authorities and institutes. The new term “permitted daily exposure” (PDE) is defined in the present guideline as a pharmaceutically acceptable intake of residual solvents to avoid confusion of differing values for ADI′s of the same substance.
Residual solvents assessed in this guideline are listed in Appendix 1 by common names and structures. They were evaluated for their possible risk to human health and placed into one of three classes as follows:
Class 1 solvents: Solvents to be avoided
Known human carcinogens, strongly suspected human carcinogens, and environmental hazards.
Class 2 solvents: Solvents to be limited
Non-genotoxic animal carcinogens or possible causative agents of other irreversible toxicity such as neurotoxicity or teratogenicity.
Solvents suspected of other significant but reversible toxicities.
Class 3 solvents: Solvents with low toxic potential
Solvents with low toxic potential to man; no health-based exposure limit is needed. Class 3 solvents have PDEs of 50 mg or more per day.
3.2. METHODS FOR ESTABLISHING EXPOSURE LIMITS
The method used to establish permitted daily exposures for residual solvents is presented in Appendix 3. Summaries of the toxicity data that were used to establish limits are published in Pharmeuropa, Vol. 9, No. 1, Supplement April 1997.
3.3. OPTIONS FOR DESCRIBING LIMITS OF CLASS 2 SOLVENTS
Two options are available when setting limits for Class 2 solvents.
Option 1
The concentration limits in parts per million stated in Table 2 can be used. They were calculated using equation (1) below by assuming a product mass of 10 g administered daily.
Here, PDE is given in terms of mg/day and dose is given in g/day.
These limits are considered acceptable for all substances, excipients, or products. Therefore this option may be applied if the daily dose is not known or fixed. If all excipients and active substances in a formulation meet the limits given in Option 1, then these components may be used in any proportion. No further calculation is necessary provided the daily dose does not exceed 10 g. Products that are administered in doses greater than 10 g per day should be considered under Option 2.
Option 2
It is not considered necessary for each component of the medicinal product to comply with the limits given in Option 1. The PDE in terms of mg/day as stated in Table 2 can be used with the known maximum daily dose and equation (1) above to determine the concentration of residual solvent allowed in a medicinal product. Such limits are considered acceptable provided that is has been demonstrated that the residual solvent has been reduced to the practical minimum. The limits should be realistic in relation to analytical precision, manufacturing capability, reasonable variation in the manufacturing process, and the limits should reflect contemporary manufacturing standards.
Option 2 may be applied by adding the amounts of a residual solvent present in each of the components of the medicinal product. The sum of the amounts of solvent per day should be less than that given by the PDE.
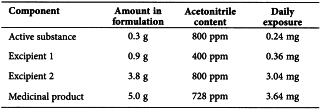
Consider an example of the use of Option l and Option 2 applied to acetonitrile in a medicinal product. The permitted daily exposure to acetonitrile is 4.1 mg per day; thus, the Option 1 limit is 410 ppm. The maximum administered daily mass of a medicinal product is 5.0 g, and the medicinal product contains two excipients. The composition of the medicinal product and the calculated maximum content of residual acetonitrile are given in the following table.
Excipient l meets the Option l limit, but the drug substance, excipient 2, and medicinal product do not meet the Option l limit. Nevertheless, the product meets the Option 2 limit of 4.l mg per day and thus conforms to the recommendations in this guideline.
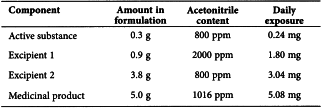
Consider another example using acetonitrile as residual solvent. The maximum administered daily mass of a medicinal product is 5.0 g, and the medicinal product contains two excipients. The composition of the medicinal product and the calculated maximum content of residual acetonitrile is given in the following table.
In this example, the product meets neither the Option 1 nor the Option 2 limit according to this summation. The manufacturer could test the medicinal product to determine if the formulation process reduced the level of acetonitrile. If the level of acetonitrile was not reduced during formulation to the allowed limit, then the manufacturer of the medicinal product should take other steps to reduce the amount of acetonitrile in the medicinal product. If all of these steps fail to reduce the level of residual solvent, in exceptional cases the manufacturer could provide a summary of efforts made to reduce the solvent level to meet the guideline value, and provide a risk-benefit analysis to support allowing the product to be utilised containing residual solvent at a higher level.
3.4. ANALYTICAL PROCEDURES
Residual solvents are typically determined using chromatographic techniques such as gas chromatography. Any harmonised procedures for determining levels of residual solvents as described in the pharmacopoeias should be used, if feasible. Otherwise, manufacturers would be free to select the most appropriate validated analytical procedure for a particular application. If only Class 3 solvents are present, a non-specific method such as loss on drying may be used.
Validation of methods for residual solvents should conform to ICH guidelines “Text on Validation of Analytical Procedures” and “Extension of the ICH Text on Validation of Analytical Procedures”.
3.5. REPORTING LEVELS OF RESIDUAL SOLVENTS
Manufacturers of pharmaceutical products need certain information about the content of residual solvents in excipients or active substances in order to meet the criteria of this guideline. The following statements are given as acceptable examples of the information that could be provided from a supplier of excipients or active substances to a pharmaceutical manufacturer. The supplier might choose one of the following as appropriate:
(Here the supplier would name the Class 2 solvents represented by X, Y, ...)
If Class 1 solvents are likely to be present, they should be identified and quantified. “Likely to be present” refers to the solvent used in the final manufacturing step and to solvents that are used in earlier manufacturing steps and not removed consistently by a validated process.
If solvents of Class 2 or Class 3 are present at greater than their Option 1 limits or 0.5 per cent, respectively, they should be identified and quantified.
4. LIMITS OF RESIDUAL SOLVENTS
4.1. SOLVENTS TO BE AVOIDED
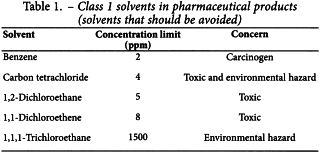
Solvents in Class 1 should not be employed in the manufacture of active substances, excipients, and medicinal products because of their unacceptable toxicity or their deleterious environmental effect. However, if their use is unavoidable in order to produce a medicinal product with a significant therapeutic advance, then their levels should be restricted as shown in Table 1, unless otherwise justified. 1,1,1-Trichloroethane is included in Table 1 because it is an environmental hazard. The stated limit of 1500 ppm is based on a review of the safety data.
4.2. SOLVENTS TO BE LIMITED
Solvents in Table 2 should be limited in pharmaceutical products because of their inherent toxicity. PDEs are given to the nearest 0.1 mg/day, and concentrations are given to the nearest 10 ppm. The stated values do not reflect the necessary analytical precision of determination. Precision should be determined as part of the validation of the method.
4.3. SOLVENTS WITH LOW TOXIC POTENTIAL
Solvents in Class 3 (shown in Table 3) may be regarded as less toxic and of lower risk to human health. Class 3 includes no solvent known as a human health hazard at levels normally accepted in pharmaceuticals. However, there are no long-term toxicity or carcinogenicity studies for many of the solvents in Class 3. Available data indicate that they are less toxic in acute or short-term studies and negative in genotoxicity studies. It is considered that amounts of these residual solvents of 50 mg per day or less (corresponding to 5000 ppm or 0.5 per cent under Option l) would be acceptable without justification. Higher amounts may also be acceptable provided they are realistic in relation to manufacturing capability and good manufacturing practice.
4.4. SOLVENTS FOR WHICH NO ADEQUATE TOXICOLOGICAL DATA WAS FOUND
The following solvents (Table 4) may also be of interest to manufacturers of excipients, active substances, or medicinal products. However, no adequate toxicological data on which to base a PDE was found. Manufacturers should supply justification for residual levels of these solvents in pharmaceutical products.
GLOSSARY
Genotoxic carcinogens Carcinogens which produce cancer by affecting genes or chromosomes.
LOEL Abbreviation for lowest-observed effect level.
Lowest-observed effect level The lowest dose of substance in a study or group of studies that produces biologically significant increases in frequency or severity of any effects in the exposed humans or animals.
Modifying factor A factor determined by professional judgement of a toxicologist and applied to bioassay data to relate that data safely to humans.
Neurotoxicity The ability of a substance to cause adverse effects on the nervous system.
NOEL Abbreviation for no-observed-effect level.
No-observed-effect level The highest dose of substance at which there are no biologically significant increases in frequency or severity of any effects in the exposed humans or animals.
PDE Abbreviation for permitted daily exposure.
Permitted daily exposure The maximum acceptable intake per day of residual solvent in pharmaceutical products.
Reversible toxicity The occurrence of harmful effects that are caused by a substance and which disappear after exposure to the substance ends.
Strongly suspected human carcinogen A substance for which there is no epidemiological evidence of carcinogenesis but there are positive genotoxicity data and clear evidence of carcinogenesis in rodents.
Teratogenicity The occurrence of structural malformations in a developing foetus when a substance is administered during pregnancy.
APPENDIX 2. ADDITIONAL BACKGROUND
A2.1. ENVIRONMENTAL REGULATION OF ORGANIC VOLATILE SOLVENTS
Several of the residual solvents frequently used in the production of pharmaceuticals are listed as toxic chemicals in Environmental Health Criteria (EHC) monographs and the Integrated Risk Information System (IRIS). The objectives of such groups as the International Programme on Chemical Safety (IPCS), the United States Environmental Protection Agency (USEPA) and the United States Food and Drug Administration (USFDA) include the determination of acceptable exposure levels. The goal is protection of human health and maintenance of environmental integrity against the possible deleterious effects of chemicals resulting from long-term environmental exposure. The methods involved in the estimation of maximum safe exposure limits are usually based on long-term studies. When long-term study data are unavailable, shorter term study data can be used with modification of the approach such as use of larger safety factors. The approach described therein relates primarily to long-term or life-time exposure of the general population in the ambient environment, i.e. ambient air, food, drinking water and other media.
A2.2.RESIDUAL SOLVENTS IN PHARMACEUTICALS
Exposure limits in this guideline are established by referring to methodologies and toxicity data described in EHC and IRIS monographs. However, some specific assumptions about residual solvents to be used in the synthesis and formulation of pharmaceutical products should be taken into account in establishing exposure limits. They are:
APPENDIX 3. METHODS FOR ESTABLISHING EXPOSURE LIMITS
The Gaylor-Kodell method of risk assessment (Gaylor, D. W. and Kodell, R. L. Linear Interpolation algorithm for low dose assessment of toxic substance. J. Environ. Pathology, 4, 305, 1980) is appropriate for Class 1 carcinogenic solvents. Only in cases where reliable carcinogenicity data are available should extrapolation by the use of mathematical models be applied to setting exposure limits. Exposure limits for Class 1 solvents could be determined with the use of a large safety factor (i.e., 10 000 to 100 000) with respect to the no-observed-effect level (NOEL). Detection and quantification of these solvents should be by state-of-the-art analytical techniques.
Acceptable exposure levels in this guideline for Class 2 solvents were established by calculation of PDE values according to the procedures for setting exposure limits in pharmaceuticals (Pharmacopeial Forum, Nov-Dec 1989), and the method adopted by IPCS for Assessing Human Health Risk of Chemicals (Environmental Health Criteria 170, WHO, 1994). These methods are similar to those used by the USEPA (IRIS) and the USFDA (Red Book) and others. The method is outlined here to give a better understanding of the origin of the PDE values. It is not necessary to perform these calculations in order to use the PDE values tabulated in Section 4 of this document.
PDE is derived from the no-observed-effect level (NOEL), or the lowest-observed effect level (LOEL), in the most relevant animal study as follows:
The PDE is derived preferably from a NOEL. If no NOEL is obtained, the LOEL may be used. Modifying factors proposed here, for relating the data to humans, are the same kind of “uncertainty factors” used in Environmental Health Criteria (Environmental Health Criteria 170, World Health Organization, Geneva, 1994), and “modifying factors” or “safety factors” in Pharmacopoeial Forum. The assumption of 100 per cent systemic exposure is used in all calculations regardless of route of administration.
The modifying factors are as follows:
| F1 | = | A factor to account for extrapolation between species |
| F1 | = | 2 for extrapolation from dogs to humans |
| F1 | = | 2.5 for extrapolation from rabbits to humans |
| F1 | = | 3 for extrapolation from monkeys to humans |
| F1 | = | 5 for extrapolation from rats to humans |
| F1 | = | 10 for extrapolation from other animals to humans |
| F1 | = | 12 for extrapolation from mice to humans |
F1 takes into account the comparative surface area: body weight ratios for the species concerned and for man. Surface area (S) is calculated as:
in which m = body mass, and the constant k has been taken to be 10. The body weight used in the equation are those shown below in Table A3.-1.
| F2 | = | A factor of 10 to account for variability between individuals. A factor of 10 is generally given for all organic solvents, and 10 is used consistently in this guideline. |
| F3 | = | A variable factor to account for toxicity studies of short-term exposure. |
| F3 | = | 1 for studies that last at least one half-lifetime (1 year for rodents or rabbits; 7 years for cats, dogs and monkeys). |
| F3 | = | 1 for reproductive studies in which the whole period of organogenesis is covered. |
| F3 | = | 2 for a 6 month study in rodents, or a 3.5 year study in non-rodents. |
| F3 | = | 5 for a 3 month study in rodents, or a 2 year study in non-rodents. |
| F3 | = | 10 for studies of a shorter duration. |
In all cases, the higher factor has been used for study durations between the time points, e.g. a factor of 2 for a 9 month rodent study.
| F4 | = | A factor that may be applied in cases of severe toxicity, e.g. non-genotoxic carcinogenicity, neurotoxicity or teratogenicity. In studies of reproductive toxicity, the following factors are used: |
| F4 | = | 1 for foetal toxicity associated with maternal toxicity |
| F4 | = | 5 for foetal toxicity without maternal toxicity |
| F4 | = | 5 for a teratogenic effect with maternal toxicity |
| F4 | = | 10 for a teratogenic effect without maternal toxicity |
| F5 | = | A variable factor that may be applied if the no-effect level was not established. |
When only a LOEL is available, a factor of up to 10 can be used depending on the severity of the toxicity.
The weight adjustment assumes an arbitrary adult human body weight for either sex of 50 kg. This relatively low weight provides an additional safety factor against the standard weights of 60 kg or 70 kg that are often used in this type of calculation. It is recognised that some adult patients weigh less than 50 kg; these patients are considered to be accommodated by the built-in safety factors used to determine a PDE. If the solvent was present in a formulation specifically intended for paediatric use, an adjustment for a lower body weight would be appropriate.
As an example of the application of this equation, consider the toxicity study of acetonitrile in mice that is summarised in Pharmeuropa, Vol. 9. No. 1, Supplement, April 1997, page S24. The NOEL is calculated to be 50.7 mg kg–1 day–l. The PDE for acetonitrile in this study is calculated as follows:
In this example,
| F1 | = | 12 to account for the extrapolation from mice to humans |
| F2 | = | 10 to account for differences between individual humans |
| F3 | = | 5 because the duration of the study was only 13 weeks |
| F4 | = | 1 because no severe toxicity was encountered |
| F5 | = | 1 because the no-effect level was determined |
The equation for an ideal gas, PV = nRT, is used to convert concentrations of gases used in inhalation studies from units of ppm to units of mg/L or mg/m3. Consider as an example the rat reproductive toxicity study by inhalation of carbon tetrachloride (molecular weight 153.84) summarised in Pharmeuropa, Vol. 9, No. 1, Supplement, April 1997, page S9.
The relationship 1000 L = 1 m3 is used to convert to mg/m3.